Carbon dioxide
Introduction
Carbon dioxide (CO2) is a significant gas that traps heat and is commonly referred to as a greenhouse gas. It is produced by burning fossil fuels, which include coal, oil, and natural gas, as well as by wildfires and other natural events like volcanic eruptions.
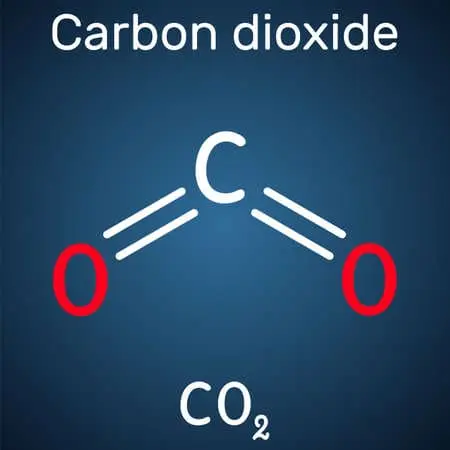
CO2 is the chemical formula for the molecule carbon dioxide. It is composed of molecules with two oxygen atoms and one carbon atom covalently doubly linked to each other.
At room temperature, atmospheric CO2 is a gas that serves as the main carbon source for life on Earth. It is also the source of accessible carbon in the carbon cycle.
While carbon dioxide in the atmosphere is invisible to visible light, it acts as a greenhouse gas by absorbing infrared radiation. Groundwater, lakes, ice caps, and oceans all contain soluble carbon dioxide.
Ocean acidification results from the dissolution of carbon dioxide in water, which mostly creates bicarbonate (HCO−3) and carbonate.
This process increases as atmospheric CO2 levels rise. At 421 parts per million (ppm), or around 0.04% (as of May 2022), it is a trace gas in the Earth’s atmosphere. Pre-industrial levels were 280 ppm, or roughly 0.025%, of this gas.
These elevated CO2 concentrations and climate change are mostly the result of burning fossil fuels. Since late in the Precambrian, animals and geological events have controlled its concentration in Earth’s pre-industrial atmosphere.
Using energy from sunlight, plants, algae, and cyanobacteria create carbohydrates from carbon dioxide and water through a process known as photosynthesis, which also yields oxygen as a byproduct.
As a result, when all aerobic organisms digest organic substances to make energy through respiration, they consume oxygen and exhale CO2 as waste.
When organic things burn or decompose, as they do in forest fires, CO2 is emitted. CO2 is essential for life on Earth since it is needed by plants for photosynthesis and by animals and people for sustenance.
Despite being 53% denser than dry air, carbon dioxide has a lengthy half-life and fully mixes with the environment. Land and ocean carbon sinks take up almost half of the excess CO2 emissions that are released into the atmosphere.
Because CO2 is released back into the atmosphere via decomposition and wildfires, these sinks are dynamic and have the potential to become saturated.
In the end, CO2 is permanently sequestered (stored) in rocks and organic deposits such as coal, oil, and natural gas.
Burning fossil fuels releases sequestered CO2 into the atmosphere, as do naturally occurring geysers, hot springs, volcanoes, and reactions between acids and water that dissolve carbonate rocks.
A versatile industrial substance, CO2 is employed, for example, as a supercritical fluid solvent in supercritical drying and coffee decaffeination, as a pressurizing gas in air cannons and oil recovery, and as an inert gas in welding and fire extinguishers.
It is a result of the fermentation of sugars used to make wine, beer, and bread. It is used to give fizz to carbonated drinks like beer and seltzer. Although it is odorless at the amounts that are typically encountered, it has a harsh, acidic smell and leaves the tongue tasting like soda water.
Chemical and Physical Properties
Structure, Bonding, and Molecular Vibrations
At its equilibrium geometry, the symmetry of a carbon dioxide molecule is centrosymmetric and linear.
The carbon-oxygen bond length in carbon dioxide is 116.3 pm, which is much less than the length of most other C–O multiply bound functional groups, such as carbonyls, and around 140 pm in a typical single C–O bond.
There is no electric dipole moment in the molecule since it is centrosymmetric. The graphic illustrates the four vibrational modes that CO2 exhibits as a linear triatomic molecule.
The atoms travel along the molecule’s axis in both the symmetric and antisymmetric stretching modes. Because of the symmetry of the molecule, there are two degenerate bending modes, which have the same frequency and energy.
The two bending modes can have distinct frequencies when a molecule comes into contact with a surface or another molecule because the two modes interact differently.
A few vibrational modes may be seen in the infrared (IR) spectrum: the degenerate pair of the antisymmetric stretching mode at wavenumber 2349 cm−1 (wavelength 4.25 μm) and. bending modes at a wavelength of 15 μm at 667 cm−1.
In Raman spectroscopy, the symmetric stretching mode is discovered at 1388 cm−1 (wavelength 7.2 μm), however, it is not recognized in infrared spectroscopy because it does not produce an electric dipole.
Carbon dioxide molecules experience strong vibrations and lose their fixed structure in the gas phase. However, an immediate image of the molecular structure may be inferred in a Coulomb explosion imaging experiment.
An experiment like this has been done with carbon dioxide. None of the molecules in the gas phase are ever precisely linear, according to the findings of this experiment and theoretical computations based on an ab initio potential energy surface of the molecule.
The reason for this seemingly contradictory outcome is insignificant as the volume factor associated with nuclear motion disappears for straight geometries. For all molecules—aside from diatomics—this is true.
In Aqueous Solution
Since carbon dioxide’s ionization in water is incomplete, it reversibly produces H2CO3, or carbonic acid, in water, where it is soluble.
CO2 + H2O ⇌ H2CO3
The carbonic acid’s hydration equilibrium constant at 25 °C is:
![{\displaystyle K_{\mathrm {h} }={\frac {{\ce {[H2CO3]}}}{{\ce {[CO2_{(aq)}]}}}}=1.70\times 10^{-3}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7b01be634ec0c3d83cbf3aa2c71bbd51b9ce0e26)
As a result, most of the carbon dioxide does not become carbonic acid but instead stays as CO2 molecules, which has no effect on pH.
The pH affects the relative amounts of CO2, H2CO3, and the deprotonated forms CO2−3 (carbonate) and HCO−3 (bicarbonate).
A Bjerrum plot illustrates how the bicarbonate form predominates (>50%) in neutral or slightly alkaline water (pH > 6.5) and becomes the most abundant (>95%) at the pH of saltwater. The most common (>50%) form in extremely alkaline water (pH > 10.4) is carbonate.
With an average pH of 8.2–8.5, the seas are quite alkaline and have a bicarbonate content of 120 mg per liter.
Because carbonic acid is diprotic, it has two acid dissociation constants. The first one is for the breakdown of the bicarbonate ion (HCO–3), commonly known as hydrogen carbonate.
H2CO3 ⇌ HCO−3 + H+
Ka1 = 2.5 × 10−4 mol/L; pKa1 = 3.6 at 25 °C.
The real first acid dissociation constant is this one, which is described as:
![{\displaystyle K_{\mathrm {a1} }={\frac {{\ce {[HCO3- ][H+]}}}{{\ce {[H2CO3]}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6013fac05dba06751345e4d824c525ed2c43b12)
When hydrated CO2(aq) is absent from the denominator and only covalently bound H2CO3 is present. The apparent amount, which is frequently
stated and much less is around 4.16 × 10−7. This number was derived under the false premise that all dissolved CO2 is present as carbonic acid.
![{\displaystyle K_{\mathrm {a1} }{\rm {(apparent)}}={\frac {{\ce {[HCO3- ][H+]}}}{{\ce {[H2CO3] + [CO2_{(aq)}]}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3ae2fa07440c037d6054ce4e7ef95c15c59264e7)
Compared to the genuine Ka1, Ka1(apparent) has a significantly bigger denominator and a much smaller value since the majority of the dissolved CO2 stays as CO2 molecules.
Depending on the pH of the solution, the bicarbonate ion, an amphoteric species, can function as either a basic or an acid. It strongly dissociates into the carbonate ion (CO2−3) at high pH:
HCO−3 ⇌ CO2−3 + H+
Ka2 = 4.69 × 10−11 mol/L; pKa2 = 10.329
The carbonic anhydrase enzyme catalyzes the synthesis of carbonic acid in living things.
Chemical Reactions of CO2
Similar in electrophilic reactivity to strong α,β-unsaturated carbonyl compounds or benzaldehyde, CO2 is a powerful electrophile.
Nucleophile reactions with CO2 are, however, thermodynamically less favorable and frequently found to be extremely reversible, in contrast to electrophiles of equal reactivity.
Carbon dioxide and amines react reversibly to form carbamates, which are employed in CO2 scrubbers and have been proposed as a potential precursor to amine gas treatment for carbon capture and storage.
Carboxylates are only produced when extremely potent nucleophiles, such as the carbanions found in Grignard reagents and organolithium compounds, combine with CO2:
MR + CO2 → RCO2M
where M = Li or Mg Br and R = alkyl or aryl.
CO2 functions as a ligand in metal carbon dioxide complexes, which can help CO2 change into other compounds.
Usually, the process of reducing CO2 to CO is challenging and slow:
CO2 + 2 e− + 2 H+ → CO + H2O
Plants and cyanobacteria, which are photoautotrophs, use solar energy to photosynthesize simple sugars from CO2 that they take from the atmosphere and water.
n CO2 + n H2O → (CH2O)n + n O2
About −0.53 V is the redox potential for this reaction in relation to the standard hydrogen electrode as it approaches pH 7. This process is catalyzed by the enzyme carbon monoxide dehydrogenase, which contains nickel.
Physical properties
Colorless is carbon dioxide. The gas has no smell at low concentrations, but when the concentration is high enough, it releases an acidic smell. The density of carbon dioxide is 1.98 kg/m3, or 1.53 times that of air, under normal temperature and pressure.
At pressures lower than 0.51795(10) MPa (5.11177 atm), carbon dioxide does not exist in a liquid form. Under 1 atm (0.101325 MPa) of pressure, the solid sublimates directly to a gas above 194.6855(30) K2 °C), and the gas deposits straight to a solid below this temperature.
Dry ice is the term used to describe carbon dioxide when it is solid. The triple point of carbon dioxide is 216.592 K (−56.558(3) °C) at 0.51795(10) MPa2 atm) (see phase diagram). Liquid carbon dioxide forms only at pressures over 0.51795(10) MPa (5.11177(99) atm).
At 7.3773(30) MPa (72.808(30) atm), or 304.128(15) K (30.978(15) °C), is the critical point. An amorphous glass-like solid has also been reported under high pressure as a type of solid carbon dioxide.
Carbonia, a kind of glass, is created by supercooling heated CO2 at pressures as high as 40–48 GPa, or over 400,000 levels) within a diamond anvil.
The idea that carbon dioxide may exist in a glass state like other elements in its elemental family, such as silicon dioxide (silica glass) and germanium dioxide, was validated by this study.
However, carbonic glass is not stable at standard pressures and turns back into gas when pressure is removed, in contrast to silica and Germania glasses.
Supercritical carbon dioxide is the name given to the behavior of carbon dioxide when it is subjected to pressures and temperatures higher than the critical point.
Table of saturated liquid carbon dioxide’s thermal and physical properties:
| Temperature (°C) | Density (kg/m3) | Specific heat (kJ/(kg⋅K)) | Kinematic viscosity (m2/s) | Thermal conductivity (W/(m⋅K)) | Thermal diffusivity (m2/s) | Prandtl Number | Bulk modulus (K^-1) |
Table of thermal and physical properties of carbon dioxide (CO2) at atmospheric pressure:
| Temperature (K) | Density (kg/m3) | Specific heat (kJ/(kg⋅°C)) | Dynamic viscosity (kg/(m⋅s)) | Kinematic viscosity (m2/s) | Thermal conductivity (W/(m⋅°C)) | Thermal diffusivity (m2/s) | Prandtl Number |
Biological Role
When an organism uses oxygen to break down carbohydrates, lipids, and amino acids for energy, it produces carbon dioxide as a byproduct of cellular respiration. This encompasses all types of plants, algae, and animals, as well as aerobic bacteria and fungi.
Vertebrates’ blood carries carbon dioxide from their bodily tissues to their skin (for example, in frogs) or gills (for example, in fish), where it dissolves in water, or to their lungs, where it is expelled.
Plants have the capacity to take in more carbon dioxide from the atmosphere during active photosynthesis than they expel during respiration.
Photosynthesis and Carbon Fixation
Through the biochemical process of carbon fixation, plants, algae, and cyanobacteria absorb carbon dioxide from the atmosphere and convert it into organic molecules that are high in energy, like glucose, facilitating photosynthesis, the process by which they make their own nourishment.
Carbon dioxide and water are used in photosynthesis to create sugars, which are the building blocks for other organic molecules.
As a byproduct, oxygen is created. As seen in the diagram on the left, ribulose-1,5-bisphosphate carboxylase oxygenase, or RuBisCO, is the enzyme responsible for the first significant step in carbon fixation.
Which is the synthesis of two molecules of 3 phosphoglycerates from CO2 and ribulose bisphosphate. It is believed that RuBisCO is the most prevalent protein on Earth.
The byproducts of photosynthesis are used by phototrophs as both internal food sources and as building blocks for the manufacture of larger, more complex organic molecules including proteins, nucleic acids, and polysaccharides.
They serve as the foundation for food chains and webs that support the growth of other creatures, including mammals like humans. The coccolithophores, a group of significant phototrophs, produce hard calcium carbonate scales.
Emiliania huxleyi is a globally significant species of coccolithophore, the calcite scales of which have formed the foundation of numerous sedimentary rocks, including limestone, allowing the fixation of atmospheric carbon over geological periods.
When compared to ambient circumstances, plants can develop up to 50% quicker under concentrations of 1,000 ppm CO2, assuming no changes in the environment and no restrictions on other nutrients.
The harvestable yield of crops improves with increasing CO2 levels; in FACE tests, wheat, rice, and soybean all showed yield increases of 12–14% with rising CO2.
Plants with higher ambient CO2 concentrations have fewer stomata growing, which lowers water consumption and increases water-use efficiency.
Research employing FACE has demonstrated that CO2 enrichment results in lower micronutrient concentrations in agricultural plants.
Since herbivores must consume more food to obtain the same quantity of protein, this might have an impact on other ecosystem components.
Under high CO2 conditions, plants can also change the content of secondary metabolites like flavonoids and phenylpropanoids.
Since most plants and algae that employ C3 photosynthesis also release CO2 during respiration, most are only net absorbers during the day.
A mature forest will create as much CO2 from respiration and the breakdown of dead specimens (fallen branches, for example) as it uses for photosynthesis in developing plants, even though a growing forest will absorb several tons of CO2 annually.
Mature forests can continue to absorb carbon and function as significant carbon sinks, defying the long-held belief that they are carbon neutral and contributing to the preservation of the Earth’s atmospheric carbon balance.
\Furthermore, and perhaps most importantly for life on Earth, photosynthesis is a process in which phytoplankton uses dissolved CO2 in the upper ocean to enhance atmospheric CO2 absorption.
Toxicity
Depending on the region, the average fresh air temperature (between sea level and 10 kPa level, or around 30 km/19 mi) above sea level) ranges from 0.036% (360 ppm) to 0.041% (412 ppm) of carbon dioxide.
According to the United Nations Economic Commission for Europe’s Globally Harmonized System of Classification and Labelling of Chemicals guidelines.
Which are based on the OECD Guidelines for the Testing of Chemicals, CO2 is an asphyxiant gas and is not categorized as dangerous or harmful. in amounts as high as 1% (10,000 ppm), causing drowsiness and a congested lung sensation in certain individuals.
Even in the presence of enough oxygen, concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause asphyxia, presenting as headache, dizziness, impaired vision and hearing, and unconsciousness in a matter of minutes to hours.
As a subset of asphyxiation, the physiological symptoms of acute carbon dioxide exposure are collectively referred to as hypercapnia.
Because gas is heavier than air, it can accumulate in sheltered or pocketed areas below average ground level, where animals may be suffocated.
This is especially true in places where gas seeps from the ground (due to subsurface volcanic or geothermal activity) in relatively high concentrations without the dispersing effects of wind. Then, carrion feeders drawn to the carcasses are eliminated as well.
Similar deaths of children have occurred close to Goma as a result of CO2 emissions from Mount Nyiragongo, a neighboring volcano.
This phenomenon is known as mazuku in Swahili. Humans adapt to higher CO2 concentrations by changing their breathing patterns and producing more bicarbonate in their kidneys to counteract the consequences of blood acidification, or acidosis.
According to several studies, 2.0 percent of inspired concentrations could be employed in enclosed environments (like submarines) because the adaptation is physiological and reversible and does not result in a five-day decline in performance or regular physical activity.
Other research, however, indicates a decline in cognitive function even at far lower doses. Furthermore, compensatory mechanisms or adaptability will not be able to rectify the persistent respiratory acidosis.
Below 1%
Studies addressing the long-term, continuous exposure of people and animals to CO2 at concentrations less than 1% are few. In the US, the maximum amount of CO2 that can be exposed at work is 0.5% (5000 ppm) for eight hours.
The International Space Station crew reported headaches, fatigue, mental slowness, emotional irritability, and disturbed sleep at this CO2 concentration.
Research conducted on rats exposed to 0.5% CO2 has shown that after eight weeks, kidney calcification and bone loss occur.
An investigation of people exposed to concentrations as low as 0.1% (1000 ppm) CO2, 2.5-hour sessions showed a substantial detrimental impact on cognitive functions. This effect is probably caused by CO2-induced increases in cerebral blood flow.
Another research found that at 1000 ppm, there was less information utilization and a decrease in fundamental activity level than at 500 ppm.
A study of the literature, however, revealed that only a tiny influence on high-level decision-making (for concentrations below 5000 ppm) was shown by a credible sample of investigations on the phenomena of carbon dioxide-caused cognitive impairment.
The majority of the research was complicated by poor study designs, comfortable environments, unknown exposure levels, and the use of several cognitive tests.
Similarly, research on the impact of CO2 concentration in motorcycle helmets has come under fire for its questionable methods, which included employing mannequins to take measurements and ignoring the self-reports of motorbike riders.
Furthermore, the CO2 content dropped to acceptable limits (0.2%) when typical motorcycle conditions were met (such as highway or city speeds) or the visor was lifted.
| Concentration | Note |
| 280 ppm | Pre-industrial levels |
| 421 ppm | Current (May 2022) levels |
| 700 ppm | ASHRAE recommendation |
| 1000 ppm | Cognitive impairment, Canada’s long-term exposure limit |
| 1000-2000 ppm | Drowsiness |
| 2000-5000 ppm | Headaches, sleepiness; poor concentration, loss of attention, and slight nausea are also possible |
| 5000 ppm | USA 8h exposure limit |
Ventilation
One of the primary reasons for high CO2 concentrations in enclosed areas, which results in poor indoor air quality, is inadequate ventilation.
Ventilation rates per person can occasionally be estimated using the carbon dioxide difference above outside concentrations under steady-state circumstances, which occur when occupancy and ventilation system operation last long enough for the CO2 concentration to stabilize.
Increased CO2 concentrations are linked to decreased performance, comfort, and health of occupants. The 2007 ASHRAE Standard 62.1 Indoor concentrations above ambient outside conditions can reach up to 2,100 parts per million depending on ventilation rates.
Therefore, with ventilation rates that satisfy this industry consensus guideline, indoor concentrations may reach 2,500 ppm if the outside concentration is 400 ppm.
Concentrations as high as 3,000 or 4,000 parts per million (ppm) have been reported in areas with inadequate ventilation.
Carbon dioxide and nitrogen mixes were referred to as “blackdamp,” “choke damp,” or “stythe” by miners, who are especially susceptible to gas exposure because of inadequate ventilation. .
Before more sophisticated technology was created, miners would sometimes bring a caged canary with them into the mine shaft to check for dangerously high levels of blackdamp and other gasses.
Because it is more susceptible to asphyxiating gasses than people are, the canary would cease singing and tumble from its perch if it fell asleep.
The Davy lamp could also identify significant concentrations of blackdamp, a gas that lowers and gathers near the floor, by dimming its light; in contrast, methane, another gas that suffocates and poses an explosive danger, would cause the lamp to brighten.
Three partygoers in Moscow perished from asphyxia in February 2020 when dry ice, or frozen carbon dioxide, was added to a swimming pool to lower its temperature.
In a comparable incident that happened in 2018, the woman died from CO2 fumes that came from the substantial amount of dry ice she was driving.
Indoor air
People are spending an increasing amount of time in enclosed spaces (between 80 and 90 percent of their time in buildings or cars).
As per multiple French actors and the French Agency for Food, Environmental and Occupational Health & Safety (ANSES), the indoor air CO2 rate of buildings.
Which is associated with animal or human occupancy and the presence of combustion installations, is typically between 350 and 2,500 parts per million (ppm) when air renewal is taken into account.
The levels of CO2 and other pollutants in residences, workplaces, schools, and nurseries do not systematically correlate, and indoor CO2 is not a reliable indicator of the pollutants associated with outside road (or air, etc.) traffic.
Along with hygrometry and oxygen levels, CO2 is the metric that fluctuates the fastest when people or animals congregate in a confined or inadequately ventilated space. Many open hearths in developing nations release CO2 and other emissions into the homes.
Outdoor Areas With Elevated Concentrations
In close proximity to powerful sources, particularly those that are secluded by surrounding topography, local concentrations of carbon dioxide can rise to high amounts.
The Bossoleto hot spring is located in a bowl-shaped depression of 100 meters (330 feet) in diameter near Rapolano Terme in Tuscany.
Italy experienced an overnight spike in CO2 concentrations exceeding 75%, which is lethal to insects and small animals.
Convection spreads the gas after daybreak. It is believed that high CO2 concentrations from disturbing deeply saturated lake water in Cameroon in 1984 killed 37 people in Lake Monoun and 1700 people in Lake Nyos in 1986.
Human Physiology
Content
Each person’s body generates around 2.3 pounds (1.0 kg) of carbon dioxide every day, which is made up of 0.63 pounds (290 g) of carbon.
Lower quantities of this carbon dioxide are found in the arteries of people because it is exhaled through the lungs and transported through the venous system.
A common way to express the blood’s carbon dioxide concentration is partial pressure, or the pressure that carbon dioxide would have had if it had taken up its whole volume. The blood carbon dioxide content in people is displayed in the table that follows.
| Blood compartment | (kPa) | (mm Hg) | |
| Venous blood carbon dioxide | 5.5–6.8 | 41–51 | 41–51 |
| Alveolar pulmonary gas pressures | 4.8 | 36 | 36 |
| Arterial blood carbon dioxide | 4.7–6.0 | 35–45 | 35–45 |
Transport in the Blood
The blood carries CO2 in three different ways. (The precise percentages differ for venous and arterial blood).
- The majority, or around 70% to 80%, is transformed into bicarbonate ions HCO−3 in red blood cells by the carbonic anhydrase enzyme by the following reaction:
CO2 + H2O → H2CO3 → H+ + HCO−3
- Blood plasma dissolves 5–10% of it.
- Hemoglobin has 5–10% carbamino molecules linked to it.
Both oxygen and carbon dioxide are carried by hemoglobin, the primary oxygen-carrying molecule in red blood cells. The CO2 attached to hemoglobin, however, does not bind to the same location as oxygen.
As an alternative, it joins forces with the four globin chains’ N-terminal groups. Nevertheless, at a given partial pressure of oxygen, the binding of CO2 reduces the quantity of bound oxygen due to allosteric effects on the hemoglobin molecule.
This process called the Haldane Effect, is crucial for moving carbon dioxide from the tissues to the lungs. On the other hand, the Bohr effect—a decrease in pH or an increase in CO2 partial pressure—causes hemoglobin to release its oxygen.
Regulation of Respiration
One of the mediators of the local autoregulation of blood supply is carbon dioxide. When its concentration is high, the capillaries widen, increasing the amount of blood that may reach that region.
The balance of bicarbonate ions in the blood must be maintained. The pace at which someone breathes affects the amount of CO2 in their blood.
Breathing too quickly can result in hyperventilation, which can induce respiratory alkalosis, whereas breathing too slowly or shallowly can produce respiratory acidosis.
Even though the body needs oxygen for metabolism, respiration is often not stimulated by low oxygen levels. Rather, increased carbon dioxide levels cause breathing to become more active.
Therefore, without ever experiencing air hunger, inhaling low-pressure air or a gas combination containing no oxygen at all (like pure nitrogen) might cause unconsciousness.
For fighter pilots flying at high altitudes, this is extremely dangerous. This is also the reason that flight attendants advise customers to put on their oxygen masks before assisting others in the event that the cabin pressure is lost; failing to do so puts them in danger of unconsciousness.
A 40 mmHg arterial CO2 pressure is the target that the respiratory centers strive to maintain. Intentional hyperventilation reduces the respiratory drive and lowers the arterial blood’s CO2 concentration to 10–20 mmHg (with minimal effect on the blood’s oxygen content).
This explains why, compared to not hyperventilating, one may hold their breath longer after hyperventilating. Hyperventilation is especially harmful prior to free diving since it bears the potential of unconsciousness before the demand for breathing becomes overpowering.
Concentrations and Role in the Environment
Atmosphere
Carbon dioxide is a trace gas found in Earth’s atmosphere that is essential to photosynthesis, the carbon cycle, the greenhouse effect, and the oceanic carbon cycle. It is one of the many greenhouse gases found in Earth’s atmosphere.
As of May 2022, the average worldwide concentration of CO2 in the atmosphere is 421 ppm, or 0.04%. This is a 50% increase from 280 ppm during the 10,000 years before to the mid-18th century when the Industrial Revolution began.
Human activity is the cause of the rise. Both climate change and these elevated CO2 concentrations are mostly the result of fossil fuel combustion.
The manufacturing of cement, deforestation, and burning of biomass are further significant human sources.
Carbon dioxide is a greenhouse gas that emits and absorbs infrared radiation at its two infrared-active vibrational frequencies while being transparent to visible light.
At wavelengths of 4.26 μm (2,347 cm−1) (asymmetric stretching vibrational mode) and 14.99 μm (667 cm−1) (bending vibrational mode), CO2 both absorbs and emits infrared light. The greenhouse effect significantly affects the temperature of the Earth’s surface.
The infrared spectrum is where Earth’s surface emits light with the greatest intensity. as opposed to light emission from the considers which is most strong in the visible area, which is between 200 and 2500 cm−1.
Warming of the surface and lower atmosphere results from the absorption of infrared light at the vibrational frequencies of atmospheric CO2 .
Because of this absorption, less energy reaches the upper atmosphere, making it colder. The observed rise in the average global temperature and ocean acidification are caused by increases in the atmospheric concentrations of CO2 and other long-lived greenhouse gases including methane, nitrous oxide, and ozone.
These gases also increase the atmosphere’s absorption and emission of infrared radiation. The CO2 fertilization impact is an additional direct effect.
Numerous indirect consequences of climate change on the natural world, ecosystems, and human cultures are brought about by these changes. More heat is caused by carbon dioxide than by all other greenhouse gases combined.
Because of the imbalance that results from the extraction and burning of fossil carbon, its atmospheric lifespan grows as The quick carbon cycle on Earth has been enforced by this activity.
This implies that long after these carbon transfer processes start to slow down, a portion (about 20–35%) of the fossil carbon transported thus far will remain in the atmosphere at high CO2 levels.
The biogeochemical cycle known as the carbon cycle involves the flow of carbon between the biosphere, soil, rocks, and seas on Earth.
Through the process of photosynthesis, plants, and other photoautotrophs use sun energy to create carbohydrates from water and carbon dioxide in the atmosphere.
The majority of other creatures rely on carbohydrates produced during photosynthesis as their main supply of carbon molecules and energy. The current CO2 content in the atmosphere is the greatest it has been in 14 million years.
Atmosphere-wide CO2 concentrations ranged from 180 ppm during the most recent two million years of the Quaternary glacier to as high as 4,000 ppm during the Cambrian era, some 500 million years ago.
The atmospheric CO2 concentrations peaked at about 2,000 parts per million during the Devonian (400 million years ago), again in the.
Triassic (220–200 million years ago), and were four times present during the Jurassic (201–145 million years ago) periods, according to temperature records that have been reconstructed for the last 420 million years.
Oceans
Ocean Acidification
In the ocean, carbon dioxide dissolves to produce carbonate (CO2−3), bicarbonate (HCO−3), and carbonic acid (H2CO3). The amount of dissolved carbon dioxide in the seas is around fifty times greater than that in the atmosphere.
The seas absorb a third of the CO2 released by human activities, making them a massive carbon sink. The continuous acidification of the oceans is the result of a declining ocean pH. The average pH of the ocean surface decreased from about 8.15 to 8.05.
This occurred between 1950 and 2020. Ocean acidification is mostly caused by human-caused carbon dioxide emissions, with atmospheric CO2 concentrations hitting 410 parts per million (ppm) by 2020.
The oceans take up CO2 from the atmosphere. As a result, carbonic acid (H2CO3) is produced, which splits into bicarbonate ions. (HCO−3) as well as an H+ hydrogen ion.
Although saltwater is still alkaline with a pH higher than 8, the presence of free hydrogen ions (H+) decreases the pH of the ocean and increases acidity.
Mollusks and corals, which are marine calcifying creatures, are particularly vulnerable because they need calcium carbonate to make their skeletons and shells.
Since the pH scale is logarithmic, a change of one pH unit is equal to a tenfold change in hydrogen ion concentration, meaning that a pH shift of 0.1 corresponds to a 26% rise in hydrogen ion concentration in the world’s oceans.
The pH and carbonate saturation levels at the sea surface differ based on the location and depth of the ocean. More CO2 may be absorbed by seas that are colder and higher in latitude.
As a result, the pH and carbonate saturation levels in these regions may decrease and acidity may increase.
Aside from sea ice coverage and ocean currents and upwelling zones, other variables affect the atmosphere-ocean CO2 exchange and, consequently.
Local ocean acidification is in proximity to big continental rivers and atmospheric interaction with nitrogen and sulfur from burning fossil fuels and agriculture.
Large-scale direct and indirect effects on species and their environments can result from changes in ocean chemistry. The process of making shells from calcium carbonate (CaCO3) is one of the most significant effects of rising ocean acidity.
Numerous marine species depend on this process, known as calcification, for their life and biology.
The process of calcification entails the precipitation of dissolved ions into solid CaCO3 structures, which are home to a variety of marine creatures including mollusks, crustaceans, foraminifera, and coccolithophores.
These CaCO3 structures are susceptible to disintegration after they are produced, unless the surrounding seawater has saturation-level concentrations of carbonate amount of additional carbon dioxide that enters the water and dissolves is quite little.
Most of it splits into free hydrogen ions and more bicarbonate. The reaction is unbalanced because the rise in hydrogen is greater than the increase in bicarbonate:
HCO−3 ⇌ CO2−3 + H+
Some of the carbonate ions that are already present in the ocean mix with some of the hydrogen ions to form more bicarbonate in order to preserve chemical balance.
Consequently, the ocean’s carbonate ion concentration decreases, eliminating a necessary component needed by marine animals to calcify, or form shells:
Ca2+ + CO2−3 ⇌ CaCO3
Hydrothermal Vents
Hydrothermal vents are another way that carbon dioxide enters the seas. As of 2004, there were only two known places in the world that produced nearly pure liquid carbon dioxide—the other being the.
Okinawa Trough—which is produced by the Champagne hydrothermal vent at the Northwest Eifuku volcano in the Mariana Trench. In 2006, news of the discovery of a liquid carbon dioxide submerged lake in the Okinawa Trough was released.
Production
Biological Processes
When sugar is fermented to make bioethanol and alcoholic drinks like beer and whiskey, carbon dioxide is produced as a byproduct. The following is how yeast breaks down sugar to create
C6H12O6 → 2 CO2 + 2 CH3CH2OH
When proteins, fatty acids, and carbohydrates are oxidized, CO2 is produced by all aerobic organisms. The several responses involved are incredibly intricate and difficult to explain.
See (anaerobic respiration, photosynthesis, and cellular respiration). The following formula describes how glucose and other monospace
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Methane and carbon dioxide are produced during the breakdown of organic matter by anaerobic microbes, along with minute amounts of other chemicals.
The kinetics of gas generation are well-defined, independent of the kind of organic material.
Approximately 40–45% of the gas released during landfill decomposition—also referred to as “landfill gas”—is carbon dioxide. Methane makes up most of the remaining 50–55%.
Industrial processes
Distillation from air can provide carbon dioxide, but the process is ineffective. In the industrial setting, carbon dioxide is primarily an unrecoverable waste product that may be generated at different sizes using a variety of techniques.
Combustion
All carbon-based fuels, including coal, wood, petroleum distillates (gasoline, diesel, kerosene, propane), methane (natural gas), and general organic matter, when they burn, release carbon dioxide and water, unless the fuel is pure carbon. The chemical interaction between methane and oxygen, for instance:
CH4 + 2 O2 → CO2 + 2 H2O
Pig iron and carbon dioxide are produced in a blast furnace when iron is reduced from its oxides with coke:
Fe2O3 + 3 CO → 3 CO2 + 2 Fe
By-Product From Hydrogen Production
The industrial processes of steam reforming to produce hydrogen and the water gas shift reaction to produce ammonia both produce carbon dioxide as byproducts.
The interaction between water and natural gas—primarily methane—starts these processes. This is a significant source of food-grade carbon dioxide.
Which is used to carbonate soft beverages and beer, as well as to shock animals like chickens. A scarcity of carbon dioxide for these uses emerged in Europe during the summer of 2018 as a result of the temporary maintenance shutdown of many ammonia factories.
Thermal Decomposition of Limestone
The process involves heating limestone, CaCO3, to around 850°C (1,560°F) in order to make quicklime, also known as calcium oxide or CO2. Quicklime is a chemical with several industrial applications.
CaCO3 → CaO + CO2
CO2 is released from most metal carbonates by acids. As a result, natural carbon dioxide springs, which generate it through the reaction of acidified water with limestone or dolomite, are a direct source of it. The following illustrates how hydrochloric acid and calcium carbonate, or chalk, react:
CaCO3 + 2 HCl → CaCl2 + H2CO3
After that, the carbonic acid (H2CO3) breaks down into CO2 and water:
H2CO3 → CO2 + H2O
As the gas is released, such reactions might cause bubbling, foaming, or both. Because they may be used to neutralize waste acid streams, they are widely employed in industry.
Commercial Uses
The food sector, the oil industry, and the chemical industry employ carbon dioxide. While the molecule has a wide range of commercial applications, the creation of carbonated beverages—such as soda water, beer, and sparkling wine—is one of its most important functions as a chemical.
Precursor to Chemicals
In the chemical industry, carbon dioxide is mostly utilized to make urea, with a minor amount also going toward making methanol and several other chemicals CO2.
Sodium salicylate is one of the derivatives of carboxylic acids that are made with CO2 by the Kolbe-Schmitt reaction.
Apart from the standard procedures that employ CO2 for chemical synthesis, researchers are now investigating electrochemical techniques.
It is particularly appealing to use renewable energy to produce fuels from CO2 (like methanol), as this might provide fuels that are easy to transport and use in traditional combustion technologies, all while producing zero net CO2 emissions.
Agriculture
For plants to engage in photosynthesis, carbon dioxide is required. Greenhouses that are huge in size may need to supplement their atmospheres with more CO2 in order to maintain and accelerate plant development.
In a greenhouse, pests like spider mites and whiteflies can be eliminated by elevating the concentration of carbon dioxide to 10,000 ppm (1%) or more over many hours. This is because the gas can be hazardous to animals at concentrations of 100 times the atmospheric concentration or higher.
Foods
In the food business, carbon dioxide is a food ingredient that is used as an acidity regulator and propellant. It is authorized for use in the US, Australia, and New Zealand, as well as the EU (listed as E number E290) (listed by its INS number 290).
Carbon dioxide gas is used to pressurize Pop Rocks candies to a pressure of around 4,000 kPa (40 bar; 580 psi).
Like other hard candies, it melts in the mouth and pops audibly to release the gas bubbles. The dough rises because leavening chemicals release carbon dioxide.
Whereas artificial leaveners like baking soda and powder emit carbon dioxide when heated or exposed to acids, baker’s yeast creates carbon dioxide through the fermentation of sugars in the dough.
Beverages
Soda water and carbonated soft drinks are made with carbon dioxide. While spontaneous fermentation has historically produced the carbonation of beer and sparkling wine, many producers now use carbon dioxide that has been captured during the fermentation process.
The most popular technique for carbonating beer that has been bottled or kegged is using recycled carbon dioxide.
Draught beer, other than true ale from Britain, is often moved from kegs in a basement or cool room to taps on the bar with the use of pressurized carbon dioxide, which is occasionally combined with nitrogen.
Rather than the gas exploding into bubbles, the flavor of soda water (and similar taste sensations in other carbonated beverages) is a result of the dissolved carbon dioxide.
A sour taste results from the conversion of carbonic anhydrase 4 to carbonic acid, and the dissolved carbon dioxide also elicits a tactile reaction.
Winemaking
In order to assist stop wild yeast from fermenting grape clusters on their own, dry ice containing carbon dioxide is frequently utilized during the cold soak stage of winemaking.
Dry ice has one major benefit over water ice: it cools the grapes without introducing more water, which might lower the grape must’s sugar content and, consequently, the wine’s alcohol content.
In order to manufacture Beaujolais wine through a procedure called carbonic maceration, a hypoxic atmosphere is also created using carbon dioxide.
Although it can dissolve into the wine and make previously still wine somewhat effervescent, carbon dioxide is occasionally used to fill off wine bottles or other storage containers like barrels to avoid oxidation.
Professional wine producers thus prefer to use other gases, such as nitrogen or argon, for this operation.
Stunning Animals
The use of carbon dioxide to “stun” animals before slaughter is common. “Stunning” could be a stretch given that the animals might experience discomfort and are not instantly rendered unconscious.
Inert gas
One of the most often utilized compressed gases for pneumatic (pressurized gas) systems in portable pressure tool applications is carbon dioxide.
Although it reacts to oxidize most metals in the welding arc, carbon dioxide is also utilized as an environment for welding.
Despite strong evidence that welds produced in carbon dioxide are more brittle than those produced in more inert atmospheres, the automobile industry nonetheless often uses these welds.
Because CO2 can react at these high temperatures, it is commonly referred to as “MAG welding,” or metal active gas, when used for MIG welding. It usually creates a puddle that is hotter than actually inert environments, which enhances the flow properties.
But this can be because of atmospheric processes that are taking place at the puddle location. When welding, this is often the reverse of what is wanted because it tends to embrittle the site.
However, with mild steel welding in general, where final ductility is not a key concern, this might not be an issue.
Because it is nonflammable, inexpensive, and undergoes a phase transition from gas to liquid at room temperature at a pressure of about 60 bar (870 psi; 59 atm), carbon dioxideis utilized in a lot of consumer goods that need pressurized gas.
This allows for far more carbon dioxide to fit in a given container than would otherwise be possible.
Canisters of pressurized carbon dioxide are frequently seen in life jackets, enabling rapid inflation. Aluminum CO2 capsules are also available for purchase as compressed gas supplies for air weapons, paintball markers, and guns.
Bicycle tire inflation, and the production of carbonated water. You may also employ high carbon dioxide concentrations to eradicate pests.
Liquid carbon dioxide finds application in the decaffeination of coffee beans, the supercritical drying of some food products and industrial materials, and the preparation of specimens for scanning electron microscopy.
Fire Extinguisher
By saturating the area surrounding the flame with carbon dioxide, fires can be put out. Instead of reacting to put out the flame on its own, it starves it of oxygen by pushing it to another location.
Certain fire extinguishers, particularly the ones made for electrical fires, include liquid carbon dioxide that is compressed.
Carbon dioxide extinguishers are effective against tiny electrical and flammable liquid fires, but they are ineffective against larger combustible flames because they do not considerably reduce the temperature.
The burning materials, and as the carbon dioxide disperses, expose the materials to ambient oxygen, which might ignite the materials.
Server rooms are the primary usage for them. In fixed fire-protection systems, carbon dioxide is also frequently utilized as an extinguishing agent to completely flood a protected area and apply localized pressure to certain risks.
Carbon dioxide systems are recognized by the International Maritime Organization as a fire safety measure for ship holds and engine rooms.
Because carbon dioxide may suffocate a person in large enough quantities, fire defense systems based on this gas have been connected to several fatalities.
51 events with CO2 systems were found between 1975 and the report’s publication date of 2000, resulting in 145 injuries and 72 fatalities.
Supercritical CO2 as Solvent
The caffeine in coffee is extracted using liquid carbon dioxide, which also works well as a solvent for a variety of lipophilic chemical substances. in the pharmaceutical and chemical processing industries.
Carbon dioxide has gained interest as a less hazardous substitute for conventional solvents like organochlorides. For this reason, certain dry cleaners also use it. Because of the characteristics of supercritical carbon dioxide, it is utilized in the creation of some aerogels.
Medical and Pharmacological Uses
Up to 5% carbon dioxide (130 times the concentration of ambient carbon dioxide) is given to oxygen in medicine to stimulate breathing following apnea and to maintain the blood’s O2/CO2 equilibrium.
An inhalable gas known as “Carbogen” may be created by combining carbon dioxide with up to 50% oxygen. This gas has several applications in research and medicine. Mofettes, or dry spas, are another medicinal use for carbon dioxide used in post-volcanic discharge.
Energy
The operating fluid of the Allam power cycle engine is supercritical CO2.
Fossil Fuel Recovery
When carbon dioxide becomes miscible with oil under supercritical conditions, it is injected into or near producing oil wells for the purpose of increased oil recovery.
This technique can boost original oil recovery by lowering residual oil saturation by 7–23% in addition to the main extraction.
In addition to acting as a pressurizing agent, it also drastically lowers the viscosity of subterranean crude oil when dissolved in it, altering the surface chemistry and allowing the oil to flow through the reservoir and into the removal well more quickly.
Large-scale pipe networks are utilized in developed oil fields to transport carbon dioxide to the injection locations.
Unlike present methods that mostly rely on the removal of water (to lower pressure) to cause the coal seam to release its trapped methane, improved coal bed methane recovery would involve pumping carbon dioxide into the coal seam to displace methane.
Bio Transformation into Fuel
One idea has been to bubble CO2 from electricity generation into ponds to encourage the development of algae, which might then be used to make biodiesel fuel.
A genetically modified strain of Synechococcus elongatus cyanobacterium is able to create isobutyraldehyde and isobutanol from CO2 during photosynthesis.
By using enzymes extracted from bacteria, researchers have created a procedure known as electrolysis that drives the chemical processes that turn CO2 into energy.
Refrigerant
Particularly in the food business, where they are used for the shipping and storage of frozen delicacies like ice cream, liquid and solid carbon dioxide are vital refrigerants.
The term “dry ice” refers to solid carbon dioxide that is utilized for minor shipments when refrigeration equipment is impractical.
Regardless of air temperature, solid carbon dioxide is always below −78.5 °C (−109.3 °F) under standard atmospheric pressure.
A refrigerant known as liquid carbon dioxide (industry nomenclature R744 or R-744) was used before dichlorodifluoromethane (R12, a chlorofluorocarbon (CFC) chemical) was employed CO2.
Because 1,1,1,2-tetrafluoromethane (R134a, a hydrofluorocarbon (HFC) molecule) is one of the primary CFC alternatives and contributes more to climate change than CO2, CO2 may see a resurgence.
The physical characteristics of CO2 are excellent for heating, cooling, and refrigeration because of their high volumetric ability to cool.
CO2 systems require very mechanically robust reservoirs and components that have previously been developed for mass production in various areas due to the requirement to work at pressures of up to 130 bars (1,900 psi; 13,000 kPa).
When it comes to car air conditioning, CO2 (R744) works better than HFC (e.g., R134a) systems in over 90% of all driving circumstances for latitudes greater than 50°.
Due to its favorable environmental characteristics (GWP of 1, non-ozone depleting, non-toxic, and non-flammable), it may eventually replace HFCs used in automobiles, supermarkets, and heat pump water heaters, among other places.
Coca-Cola has used CO2-powered beverage coolers, and CO2 refrigeration and heating technology are of interest to the U.S. Army.
Minor Uses
One of the first types of lasers is the carbon dioxide laser, which uses carbon dioxide as its lasing medium. By continually adding gas to the water, carbon dioxide may be used to manage the pH of swimming pools and prevent it from increasing.
One benefit of this is that handling (more dangerous) acids is avoided. In a similar vein, it is also employed in the upkeep of reef aquariums.
Where it is frequently employed in calcium reactors to momentarily reduce the pH of water flowing over calcium carbonate to enable the latter to dissolve into the water more readily and be utilized by certain corals for the skeleton-building process.
Used as the main coolant in the British sophisticated gas-cooled nuclear power reactor. The most prevalent method for ending the lives of laboratory study animals is carbon dioxide induction.
Animals can be given CO2 by either being exposed to a steadily rising CO2 concentration or being put straight into a closed, prefilled container that contains CO2.
For the humane death of tiny rodents, the American Veterinary Medical Association’s 2020 standards for carbon dioxide induction specify that a displacement rate of 30–70% of the chamber or cage capacity per minute is ideal.
Varied species have varied CO2 percentages, which are determined by the best percentages to reduce discomfort. Additionally, carbon dioxide is utilized in a number of associated surface preparation and cleaning methods.
History of Discovery
The first gas to be identified as a distinct entity was carbon dioxide. The Flemish scientist Jan Baptist van Helmont noticed around 1640 or thereabouts that the mass of the ash he produced from burning charcoal in a closed jar was significantly less than the mass of the initial charcoal.
The remainder of the charcoal, according to his understanding, had been transformed into an unseen substance that he called a “gas” (derived from the Greek word “chaos”) or “wild spirit” (spiritus sylvestris).
The Scottish physician Joseph Black conducted more research on the characteristics of carbon dioxide in the 1750s.
He discovered that a gas he named “fixed air” could be produced by heating or treating calcium carbonate, or limestone. He saw that neither flame nor animal life could exist in the fixed air, which was denser than air.
Black also discovered that calcium carbonate will precipitate when bubbled through limewater, a saturated aqueous solution of calcium hydroxide.
He exploited this phenomenon to demonstrate how microbial fermentation and mammalian respiration both create carbon dioxide.
In 1772The method of producing carbon dioxide by dripping sulfuric acid (also known as oil of vitriol) on chalk and forcing the gas to dissolve by agitating a bowl of water in contact with the gas was described by English chemist Joseph Priestley in a paper titled Impregnating Water with Fixed Air.
The first liquefaction of carbon dioxide (at high pressures) was accomplished in 1823 by Humphry Davy and Michael Faraday.
The French inventor Adrien-Jean-Pierre Thilorier gave the first account of solid carbon dioxide (dry ice) in 1835 when he opened a pressurized container of liquid carbon dioxide and discovered that a “snow” of solid CO2 had formed due to the quick evaporation of the liquid.
In the past, carbon dioxide and nitrogen together were referred to as “blackdamp,” “stythe,” or “choke damp.” It was also encountered in well sinking and mining activities.
A suffocating combination of carbon dioxide and nitrogen was produced by biological activities and the slow oxidation of coal.
See Also
- Arterial blood gas test: This test measures the concentrations of certain dissolved gases in blood drawn from an artery.
- Bosch reaction: a metallic catalyst is used to convert CO2 and hydrogen into elemental carbon.
- Carbon dioxide removal is the process by which human activity removes carbon dioxide from the atmosphere.
- List of power plants with the lowest carbon efficiency
- list of nations ranked by emissions of carbon dioxide
- A lake that is permanently stratified and has non-intermixing water strata is known as a meromictic lake.
- Canadian physicist Gilbert Plass (1920–2004): pioneering work on CO2 and climate change
- The methanation of carbon dioxide with hydrogen is known as the Sabatier reaction.
- NASA’s climate satellite Orbiting Carbon Observatory 2
- Earth observation satellite: Greenhouse Gases Observing Satellite
- The exchange of gases between plant roots and the atmosphere is known as soil gas.
FAQ
What is called carbon dioxide?
One carbon (C) and two oxygen (O) atoms make up carbon dioxide, or CO2, a transparent gas that is frequently shortened. One of the numerous compounds on Earth where carbon is frequently found is carbon dioxide. It is stable, inert, and non-toxic under normal temperature and pressure settings. It also doesn’t burn.
What is carbon dioxide used for?
In addition to being a refrigerant, carbon dioxide is also employed in fire extinguishers, inflatable life jackets and rafts, coal-blasting, foaming rubber and plastic, encouraging plant growth in greenhouses, immobilizing animals before slaughter, and in carbonated drinks.
Where is carbon dioxide found?
Because they exhale carbon dioxide as waste, most animals are natural suppliers of the gas. Burning coal, oil, or natural gas is one of the main human energy-producing activities that results in carbon dioxide emissions.
Is carbon dioxide harmful to humans?
Inhaling CO2 is regarded as having a low level of toxicity. Because CO2 behaves as a basic asphyxiant, these are the main negative health impacts that it causes. A gas that decreases or replaces the natural oxygen in breathing air is referred to as a simple asphyxiant. Mild CO2 exposure symptoms might include fatigue and headaches.
What are carbon dioxide examples?
When carbon dioxide is exhaled from the body, it is a consequence of regular cell activity. Burning wood and fossil fuels like coal, natural gas, and gasoline also releases CO2.
How carbon dioxide is formed?
Both natural (from volcanoes, animal breath, and plant decomposition) and man-made (from burning fossil fuels like coal, oil, and natural gas to produce electricity) sources produce carbon dioxide (CO2).
Why carbon dioxide?
One significant greenhouse gas that contributes to the retention of heat in our atmosphere is carbon dioxide. Our world would be quite chilly without it. But other components of Earth’s climate are being disrupted by rising average global temperatures brought on by an increase in CO2 concentrations in our atmosphere.