Metabolism
What is a Metabolism?
Metabolism is the collection of chemical processes that support life in organisms. The three primary purposes of metabolism are: converting food into the building blocks of proteins, lipids, nucleic acids, and some carbohydrates; removing metabolic wastes from the body; and converting dietary energy into energy needed to power cellular operations.
These enzyme-catalyzed processes enable living things to proliferate, retain their structural integrity, and react to their surroundings.
Additionally, the word “metabolism” can also refer to the totality of chemical events that occur within living organisms, including material transport into and out of cells and digestion. In this instance, the set of reactions that are described above within the cells is referred to as intermediary (or intermediate) metabolism.
One may classify metabolic processes as either anabolic, which is the synthesis of new compounds, or catabolic, which is the breakdown of existing compounds (such as glucose to pyruvate via cellular respiration) (such as proteins, carbohydrates, lipids, and nucleic acids).
Energy is often released during catabolism and consumed during anabolism. The chemical processes involved in metabolism are arranged into metabolic pathways, where a certain molecule is changed into another chemical through a number of stages, each of which is aided by an enzyme.
Enzymes are essential to metabolism because, via linking them to spontaneous events that release energy, they enable organisms to drive desired reactions that need energy and will not happen on their own.
Enzymes function as catalysts, accelerating reactions, and they also enable the control of metabolic reaction rates, for instance in response to environmental shifts or messages from other cells.
Which chemicals are harmful and which are nourishing to a given organism depends on its metabolic system. As an illustration, some prokaryotes consume hydrogen sulfide, a chemical that is toxic to vertebrates.
All of these chemical processes need energy, which is measured by an organism’s basal metabolic rate. The surprising consistency of core metabolic pathways across very diverse organisms is a characteristic of metabolism.
For instance, all known creatures have a set of carboxylic acids well known as the intermediates in the citric acid cycle. These species range from massive multicellular organisms like elephants to the unicellular bacteria Escherichia coli.
These parallels in The longevity of metabolic pathways are probably owing to their effectiveness, and their early emergence in evolutionary history is probably the reason for this.
Normal metabolism is disturbed in a number of disorders, including cancer, metabolic syndrome, and type II diabetes. Moreover, the metabolism of cancer cells differs from that of normal cells; these variations can be utilized to identify potential targets for cancer therapy.
Key Biochemicals
Four fundamental kinds of molecules—amino acids, carbohydrates, nucleic acids, and lipids—make up the majority of the structural components of plants, animals, and microorganisms.
The synthesis of these molecules during the development of cells and tissues, or their breakdown and use for energy through digestion, are the two main metabolic events that occur because these molecules are essential to life.
Polymers like DNA and proteins, which are vital macromolecules for life, may be created by combining these biochemicals.
Amino Acids And Proteins
Amino acids are linked together by peptide bonds to form a linear chain that makes up proteins. Many proteins function as enzymes, facilitating the chemical processes involved in metabolism.
Some proteins, including those that make up the cytoskeleton, a network of scaffolding that keeps cells shaped, have structural or mechanical purposes.
In addition, proteins play a crucial role in immunological responses, cell adhesion, active transport across membranes, and cell signaling.
By offering a carbon source for entrance into the citric acid cycle, amino acids also aid in the metabolism of cellular energy. This is particularly useful when glucose, or another major energy source, is in short supply or when cells are under metabolic stress.
Lipids
Of all the biochemicals, lipids are the most varied. Their primary structural applications are as constituents of internal and exterior biological membranes, including cell membranes. You can also exploit their chemical energy.
Polymers of fatty acids, known as lipids, have a long, non-polar hydrocarbon chain with a tiny, oxygen-containing polar area. Lipids are biological molecules that are either hydrophobic or amphipathic, yet they dissolve in organic solvents like ethanol, benzene, or chloroform.
The fats include a vast category of substances made up of glycerol and fatty acids; a triacylglyceride is a glycerol molecule joined to three fatty acids by ester bonds.
There are several variants of this fundamental structure, such as hydrophilic groups like phosphate found in phospholipids and backbones like sphingosine in sphingomyelin. Another important family of lipids is steroids, like steroids.
Carbohydrates
With several hydroxyl groups bound to them, carbohydrates are aldehydes or ketones that can exist as straight chains or rings.
The most prevalent biological molecule, carbohydrates serve a variety of purposes including energy storage and transportation as well as serving as structural elements. Glucose, fructose, and galactose are the three main monosaccharides that makeup carbohydrates.
There are essentially many ways in which monosaccharides can be joined to create polysaccharides.
Nucleotides
Nucleotide polymers make up the two nucleic acids, DNA and RNA. A phosphate is joined to a ribose or deoxyribose sugar group that is joined to a nitrogenous base to form each nucleotide.
For the storage, use, and interpretation of genetic information through the processes of transcription and protein biosynthesis, nucleic acids are essential.
DNA replication spreads this information and provides protection through DNA repair processes. Reverse transcription is used by many viruses with RNA genomes, including HIV, to produce its viral RNA genome’s DNA template.
Because it may catalyze chemical processes, RNA in ribozymes like spliceosomes and ribosomes functions similarly to enzymes. By joining a nucleobase to a ribose sugar, individual nucleosides are created.
These bases, which are categorized as purines or pyrimidines, are heterocyclic rings that include nitrogen. Additionally, in metabolic group transfer processes, nucleotides function as coenzymes.
Coenzymes
Although there are many different kinds of chemical processes that occur during metabolism, most of them are related to the transfer of atoms’ functional groups and their interactions within molecules.
Because of their shared chemistry, cells are able to transfer chemical groups across various processes using a limited number of metabolic intermediates. Coenzymes are these group-transfer intermediaries.
A specific coenzyme, which serves as the substrate for a number of enzymes that make and a number of enzymes that consume it, catalyzes each class of group-transfer processes. As a result, these coenzymes are produced, used, and then recycled continually.
As the cellular energy currency, adenosine triphosphate (ATP) is one essential coenzyme. Between several chemical processes, this nucleotide is utilized to transport chemical energy.
Although there isn’t much ATP in cells, the human body may utilize its own weight in ATP every day since it is constantly being repaired. Catabolism and anabolism are connected via ATP. Chemicals are broken down by catabolism and assembled by anabolism.
ATP is produced by catabolic processes and used up by anabolic ones. Furthermore, in phosphorylation processes, it acts as a transporter of phosphate groups. A vitamin is a naturally occurring substance that is small-scaled and not produced by cells.
When employed in cells, all water-soluble vitamins undergo phosphorylation or coupling to nucleotides, so fulfilling the role of coenzymes in human nutrition. Nicotinamide adenine dinucleotide (NAD+) is a crucial coenzyme that functions as a hydrogen acceptor.
It is derived from vitamin B3 (niacin). Numerous distinct kinds of Dehydrogenases convert NAD+ into NADH by removing electrons from their substrates.
All of the cell’s reductases that need the transfer of hydrogen atoms to their substrates can use this reduced version of the coenzyme as a substrate.
In the cell, nicotinamide adenine dinucleotide is found in two related forms: NADPH and NADH. Whereas NADP+/NADPH is utilized in anabolic activities, the NAD+/NADH form is more significant in catabolic reactions.
Mineral and Cofactors
Some inorganic elements are plentiful in the body, whereas others are only present in trace amounts and play important functions in metabolism.
Elements such as carbon, nitrogen, calcium, sodium, chlorine, potassium, hydrogen, phosphorus, oxygen, and sulfur make up around 99% of an individual’s body weight.
Most of the oxygen and hydrogen are found in water, while the bulk of carbon and nitrogen are found in organic substances (proteins, lipids, and carbohydrates). Electrolytes are provided by the plentiful inorganic elements.
The most significant ions are bicarbonate, an organic ion, calcium, magnesium, sodium, potassium, chloride, and phosphate.
The preservation of exact ion gradients across cell membranes is responsible for maintaining pH and osmotic pressure. Ions are also essential for the proper operation of nerves and muscles because the electrolyte exchange between the extracellular fluid and the cell’s fluid generates action potentials in these tissues’ cytoplasm.
Through ion channels, which are proteins found in the cell membrane, electrolytes enter and exit cells. For instance, calcium, sodium, and potassium must flow via ion channels in the cell membrane and T-tubules in order for muscles to contract.
Zinc and iron are the most prevalent transition metals, which are often found in organisms as trace components. While enzyme cofactors can undergo modifications during catalysis, they always revert to their initial state at the conclusion of the catalyzed process.
Metal cofactors are firmly linked to certain locations in proteins. By binding to storage proteins like ferritin or metallothionein when not in use, metal micronutrients are taken up by certain transporters within organisms.
Catabolism
The group of metabolic activities known as catabolism breaks down big molecules. Among these include the oxidation and breakdown of dietary molecules. The energy and materials required for anabolic processes, which create molecules, are supplied by the catabolic reactions.
The specifics of these catabolic processes vary from creature to organism; the chart below illustrates how different types of organisms may be categorized according to the main nutritional groups they belong to carbon, hydrogen, and sources of energy.
Organotrophs utilize organic molecules as a source of hydrogen atoms or electrons, whereas lithotrophs utilize inorganic substrates. While phototrophs use light to produce chemical energy, the movement of electrons from reduced donor molecules to oxygen, nitrate, or sulfate.
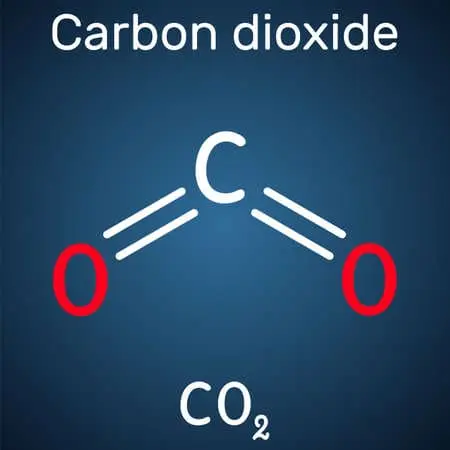
These donor molecules might be organic molecules, hydrogen, hydrogen sulfide, or ferrous ions. These processes occur in organisms when complex organic compounds are broken down into simpler molecules like water and carbon dioxide.
Similar electron-transfer processes are used by photosynthetic organisms, such as cyanobacteria and plants, to store energy from sunshine. There are three primary phases to the most prevalent collection of catabolic processes in animals.
Large organic molecules, including lipids, proteins, and polysaccharides, are broken down into smaller parts outside of cells during the first stage.
Subsequently, cells absorb these smaller molecules and transform them into smaller molecules, often acetyl coenzyme A (acetyl-CoA), which releases a small amount of energy.
Ultimately, in the citric acid cycle and electron transport chain, the acetyl group on acetyl-CoA is oxidized to water and carbon dioxide, releasing more energy and converting the coenzyme nicotinamide adenine dinucleotide (NAD+) into NADH.
Digestion
There are three primary phases to the most prevalent collection of catabolic processes in animals. Large organic molecules, including lipids, proteins, and polysaccharides, are broken down into smaller parts outside of cells during the first stage.
Subsequently, cells absorb these smaller molecules and transform them into smaller molecules, often acetyl coenzyme A (acetyl-CoA), which releases a small amount of energy.
Ultimately, in the citric acid cycle and electron transport chain, the acetyl group on acetyl-CoA is oxidized to water and carbon dioxide, releasing more energy and converting the coenzyme nicotinamide adenine dinucleotide (NAD+) into NADH.
Animals only produce digestive enzymes from specific cells in their stomachs, such as the stomach, pancreas, and salivary glands, whereas microbes just secrete these enzymes into their environment.
Active transport proteins subsequently carry the sugars or amino acids that these extracellular enzymes release into the cells.
Energy From Organic Compounds
Catabolism of carbohydrates is the process by which they break down into smaller parts. Once carbohydrates have been broken down into monosaccharides, they are typically taken up by cells.
Once inside, the primary pathway for breakdown is glycolysis, which produces some ATP and converts carbohydrates like fructose and glucose to pyruvate.
Although pyruvate is an intermediary in a number of metabolic processes, most of it is fed into the citric acid cycle and transformed into acetyl-CoA via aerobic glycolysis. Even while the citric acid cycle produces a little extra ATP, the majority of.
An essential byproduct of the oxidation of acetyl-CoA is NADH, which is produced from NAD+. Carbon dioxide is a waste product of this oxidation. Lactate is produced during anaerobic glycolysis when the enzyme lactate dehydrogenase reoxidizes NADH to NAD+ for glycolysis.
The pentose phosphate pathway, which lowers the coenzyme NADPH and generates pentose sugars like ribose, the sugar component of nucleic acids, is an alternate method for the breakdown of glucose.
Hydrolysis of fats releases free fatty acids and glycerol. Acetyl-CoA is produced when the beta-oxidation of the fatty acids breaks down the glycerol and enters glycolysis. This product is subsequently supplied into the citric acid cycle.
When oxidized, fatty acids release more energy than carbs. Certain bacteria also break down steroids in a manner akin to beta-oxidation, which results in the large release of acetyl-CoA. pyruvate, propionyl-CoA, and other substances that the cell can utilize as fuel.
As a solitary carbon source, M. tuberculosis can also thrive on lipid cholesterol, and genes linked to the cholesterol-use pathway(s) have been shown to be significant at different phases of the infection lifecycle.
Amino acids can be converted to urea and carbon dioxide for energy production, or they can be utilized to make proteins and other biomolecules. A transaminase’s removal of the amino group initiates the oxidation process.
A deaminated carbon skeleton in the form of a keto acid is left behind after the amino group is supplied into the urea cycle.
A few of these keto acids, such as α-ketoglutarate that is produced by deaminating glutamate, and are intermediates in the citric acid cycle. Gluconeogenesis, which is covered below, is another method by which glucogenic amino acids can be transformed into glucose.
Energy Transformations
Oxidative Phosphorylation
The process of oxidative phosphorylation involves the transfer of electrons from organic molecules, such as those in the citric acid cycle, to oxygen. The energy so released is then utilized to create ATP.
In eukaryotes, a group of proteins known as the electron transport chain in the mitochondrial membranes carry out this function.
These proteins are located in the inner membrane of prokaryotes. These proteins pump protons across a membrane using the energy from reduced molecules such as NADH.
An electrochemical gradient is produced and a difference in proton concentration is created across the membrane when protons are pumped out of the mitochondria.
Protons are forced back into the mitochondrion by this force via the base of the enzyme ATP synthase. The stalk subunit rotates due to the passage of protons, which causes the synthase domain’s active site to alter shape and phosphorylate adenosine diphosphate, converting it into ATP.
Energy From Inorganic Compounds
Prokaryotes have a kind of metabolism called chemolithotrophy, in which energy is produced by oxidizing inorganic substances.
These organisms can obtain energy via the oxidation of reduced sulfur compounds (such as sulfide, hydrogen sulfide, and thiosulfate), ammonia, ferrous iron, or hydrogen as sources of reducing power.
These microbial activities are essential for soil fertility and play a significant role in global biogeochemical cycles such as acetogenesis, nitrification, and denitrification.
Energy From Light
Plants, cyanobacteria, purple bacteria, green sulfur bacteria, and some protists all absorb solar energy. This process is frequently linked to photosynthesis, which is covered below, which transforms carbon dioxide into organic molecules.
Prokaryotes, on the other hand, have the ability for both the energy capture and carbon fixation systems to function independently.
This is because green sulfur bacteria and purple bacteria can both use sunlight as a source of energy and alternate between fermenting organic molecules and fixing carbon.
Since it includes the storing of energy as a gradient in the concentration of protons, the process of capturing solar energy is fundamentally comparable to oxidative phosphorylation in many organisms.
The synthesis of ATP is then propelled by this proton motive force. The light-gathering proteins known as photosynthetic reaction centers provide the electrons required to power this electron transport network.
Depending on the kind of photosynthetic pigment present, reaction centers are divided into two groups; plants and cyanobacteria have two types, whilst the majority of photosynthetic bacteria only have one.
Photosystem II in plants, algae, and cyanobacteria uses light energy to take electrons out of water, releasing waste oxygen. After that, the electrons go to the cytochrome b6f complex, which pumps protons across the chloroplast’s thylakoid membrane using its energy.
As previously, these protons drive the ATP synthase by moving back through the membrane. After passing through photosystem I, the electrons can be used to lower the coenzyme NADP+. This coenzyme can be recycled for more ATP synthesis or it can join the Calvin cycle, which is covered below.
Anabolism
The series of beneficial metabolic activities known as anabolism employ the energy liberated during catabolism to create complex molecules.
Generally speaking, smaller and simpler precursors are used to build the complex molecules that comprise cellular structures piece by piece.
Three fundamental phases comprise anabolism. Precursors such as amino acids, monosaccharides, isoprenoids, and nucleotides are synthesized in the first stage.
The second is the use of ATP energy to activate these precursors into reactive forms; and the third is the assembly of these precursors into complex molecules like proteins, polysaccharides, lipids, and nucleic acids.
Anabolism in organisms can vary depending on where the molecules in their cells come from. Autotrophs, like plants, are able to assemble basic chemicals like carbon dioxide and water into complex organic compounds like proteins and polysaccharides.
Conversely, in order to create these complex compounds, heterotrophs need a supply of more complicated materials like monosaccharides and amino acids.
The final source of energy used by an organism may be used to further classify it: photoautotrophs and photoheterotrophs derive their energy from light, whereas chemoautotrophs and chemoheterotrophs derive their energy from oxidation processes.
Carbon Fixation
The process of making carbohydrates from carbon dioxide (CO2) and sunshine is known as photosynthesis. Oxygenic photosynthesis divides water in plants, cyanobacteria, and algae; as a waste product, oxygen is created.
This mechanism converts CO2 into glycerate 3-phosphate, which can then be transformed into glucose, using the ATP and NADPH generated by the photosynthetic reaction centers, as previously mentioned.
The RuBisCO enzyme is responsible for this carbon-fixation process, which is a component of the Calvin-Benson cycle.
Three different forms of photosynthesis occur in plants, including CAM photosynthesis, C3 and C4 carbon fixation. The way carbon dioxide enters the Calvin cycle varies between these: C3 plants fix CO2 immediately, whereas C4 and CAM photosynthesis first integrate CO2 into other chemicals as a means of coping with dry, strong sunshine.
The processes of carbon fixation in photosynthetic prokaryotes are more varied. Here, acetyl-CoA can be carboxylated, the Calvin-Benson cycle reversed, or the citric acid cycle fixed to fix carbon dioxide.
Through the Calvin-Benson cycle, prokaryotic chemoautotrophs fix CO2 as well, but they get their energy from inorganic substances.
Carbohydrates And Glycans
Simple organic acids can be transformed into monosaccharides, like glucose, and then utilized to construct polysaccharides, like starch, in the process of carbohydrate anabolism.
Gluconeogenesis is the process by which glucose is produced from substances such as pyruvate, lactate, glycerol, glycerate 3-phosphate, and amino acids.
Pyruvate is converted to glucose-6-phosphate via gluconeogenesis via a sequence of intermediates, many of which are also involved in glycolysis. Nevertheless, since non-glycolytic enzymes catalyze a number of stages in this system, it is not just glycolysis done backward.
This is significant because it permits the regulation of glucose synthesis and breakdown independently and keeps the two routes from operating concurrently in an ineffective cycle.
While fat is a popular energy storage material, vertebrates, including humans, lack the enzymatic machinery essential to transform the fatty acids in these reserves into glucose through gluconeogenesis.
Plants, on the other hand, have the ability to convert acetyl-CoA into pyruvate. Therefore, in order to replenish glucose in organs like the brain that are unable to metabolize fatty acids, vertebrates must synthesize ketone bodies from fatty acids after prolonged deprivation.
Other creatures, including bacteria and plants, use the glyoxylate cycle to handle this metabolic issue. It avoids the citric acid cycle’s decarboxylation stage and enables acetyl-CoA to be converted to oxaloacetate, which may then be utilized to produce glucose.
Other than fat, most tissues store glucose as an internal energy source called glycogenesis, which is mostly utilized to keep blood glucose levels stable.
Polysaccharides and glycans are formed by glycosyltransferase adding monosaccharides one after the other to an acceptor hydroxyl group on the developing polysaccharide. This is done using a reactive sugar-phosphate donor such as uridine diphosphate glucose (UDP-Glc).
Because any hydroxyl group on the substrate’s ring can function as an acceptor, both straight and branched polysaccharides can be generated.
The resulting polysaccharides can be attached to lipids and proteins by enzymes known as oligosaccharyltransferases, or they might have structural or metabolic roles of their own.
Fatty Acids, Isoprenoids And Sterol
Acetyl-CoA units are polymerized and then reduced by fatty acid synthases to produce fatty acids.
A series of processes that add the acyl group, reduce it to an alcohol, dehydrate it to an alkene group, and then reduce it again to an alkane group lengthens the acyl chains in the fatty acids.
The enzymes involved in fatty acid biosynthesis are classified into two groups: in bacteria and plant plastids, distinct type II enzymes carry out each step of the route, but in animals and fungi, a single multifunctional type I protein is responsible for all the events involved in fatty acid synthase.
The greatest class of naturally occurring plant compounds is made up of terpenes and isoprenoids, which are a broad family of lipids that also contain carotenoids.
Isoprene units provided from the reactive precursor’s isopentenyl pyrophosphate and dimethylallyl pyrophosphate are assembled and modified to create these molecules. There are several methods for producing these precursors.
These chemicals are produced from acetyl-CoA via the mevalonate pathway in mammals and archaea, and by the non-mevalonate pathway in plants and bacteria.
Sterol biosynthesis is a significant process that makes use of these activated isoprene donors. The isoprene units are combined to produce squalene in this instance, and lanosterol is created by folding the squalene units into a set of rings.
Then, lanosterol can be transformed into various sterols, including ergosterol and cholesterol.
Proteins
The 20 common amino acids are not all synthesized by all organisms. Nine necessary amino acids must be supplied from food, while mammals can only manufacture eleven nonessential amino acids. The majority of microorganisms and plants can synthesize all twenty.
Simple parasites that obtain their amino acid needs straight from their hosts include the bacterium Mycoplasma pneumoniae, which is devoid of the ability to synthesize any amino acids.
The pentose phosphate route, the citric acid cycle, or glycolysis intermediates are used to synthesize all amino acids. Glutamate and glutamine are sources of nitrogen. To synthesize nonessential amino acids, the correct alpha-keto acid must first develop.
This alpha-keto acid is then transaminated to produce an amino acid. Proteins are created by joining amino acids together via peptide bonds.
The specific configuration of amino acid residues inside each protein determines its fundamental structure.
Amino acids may be joined in different sequences to generate a vast diversity of proteins, much as alphabet letters can be combined to form an almost infinite variety of words.
Amino acids that have attached to a transfer RNA and been active are the building blocks of proteins. by use of an ester bond. Aminoacyl tRNA synthetase catalyzes an ATP-dependent process that yields this aminoacyl-tRNA precursor.
The ribosome uses the sequence information in a messenger RNA to bind the amino acid onto the elongating protein chain, and this aminoacyl-tRNA serves as its substrate.
Nucleotide Synthesis And Salvage
A substantial quantity of metabolic energy is needed in the processes that create nucleotides from amino acids, carbon dioxide, and formic acid. As a result, most organisms have effective mechanisms in place to recover preformed nucleotides.
Nucleosides are the product of purine synthesis. Adenine and guanine are derived from the precursor nucleoside inosine monophosphate.
Which is produced by transferring formate from the coenzyme tetrahydrofolate and atoms from the amino acids glycine, glutamine, and aspartic acid. If glutamine and aspartate combine to generate the base orotate, pyrimidines are made from the latter.
Xenobiotics And Redox Metabolism
Since they lack a metabolic process, all organisms are continuously exposed to substances that they cannot utilize as food and that would be hazardous if they accumulated in cells. We refer to these potentially harmful substances as xenobiotics.
A group of enzymes that break down xenobiotics, including manufactured pharmaceuticals, natural toxins, and antibiotics, detoxify xenobiotics. These include glutathione S-transferases, UDP-glucuronosyltransferases, and cytochrome P450 oxidases in humans.
The xenobiotic is initially oxidized by this enzyme system, which then conjugates water-soluble groups onto the molecule in three phases.
After that, the altered water-soluble foreign substance may be pushed out of cells and into Before being expelled, multicellular organisms may undergo further metabolization.
The microbial biodegradation of contaminants and the bioremediation of polluted land and oil spills are two ecological processes that greatly depend on these responses.
Many of these microbial reactions are similar to those of multicellular organisms, but because of the extraordinary diversity of microbes, these organisms can break down even persistent organic pollutants like organochloride compounds and can deal with a far wider range of xenobiotics than multicellular organisms.
Oxidative stress is a related issue for species that are aerobic. Reactive oxygen species like hydrogen peroxide are created in this situation by oxidative phosphorylation and the disulfide bond synthesis that occurs during protein folding.
Antioxidant metabolites like glutathione peroxidases and catalases eliminate these harmful oxidants.
Thermodynamics Of Living Organisms
The rules of thermodynamics, which govern the movement of heat and work, must be followed by living things. According to the second rule of thermodynamics, there can never be a reduction in entropy, or disorder, in any isolated system.
Since all living things are open systems that interchange matter and energy with their environment, life is conceivable even when the incredible complexity of living things seems to defy this law.
Instead of being in equilibrium, living systems are dissipative systems that increase the entropy of their surroundings to sustain their high level of complexity.
This is accomplished via the metabolism of a cell, which links the non-spontaneous anabolic activities with the spontaneous catabolic processes. Thermodynamically speaking, metabolism creates disorder in order to preserve order.
Regulation And Control
Since most organisms’ environs are ever-changing, metabolism’s reactions need to be carefully controlled in order to preserve homeostasis, the stable state of circumstances inside cells.
Additionally, metabolic control enables organisms to actively engage with their surroundings and respond to signals. Comprehending the regulation of metabolic pathways requires a comprehension of two closely related ideas.
First, an enzyme’s ability to adjust its activity in response to inputs is known as regulation within a pathway. Furthermore, this enzyme’s control is the result that The pathway’s total rate is impacted by these variations in its activity.
An enzyme is not engaged in the regulation of a metabolic route, for instance, even if it exhibits significant activity fluctuations that have no influence on the pathway’s flow. The control of metabolism occurs on several levels.
When a substrate or product level varies, the metabolic route self-regulates to adapt. For instance, when a product level falls, the metabolic pathway’s flux may rise to make up for it.
This is known as intrinsic regulation. Often, this kind of control entails allosteric modulation of the actions of many enzymes in the route. In a multicellular organism, extrinsic control refers to a cell’s ability to alter its metabolism in response to signals from other cells.
These signals are often interpreted by certain cell surface receptors as water-soluble messengers, such as growth factors and hormones.
Second messenger systems, which frequently entail the phosphorylation of proteins, subsequently carry out the transmission of these signals inside the cell.
The way the hormone insulin controls glucose metabolism is a well-known example of extrinsic control. An increase in blood glucose levels triggers the production of insulin.
When a hormone binds to a cell’s insulin receptor, a series of protein kinases are triggered, causing the cell to absorb glucose and transform it into molecules that can be stored, such as glycogen and fatty acids.
The activity of the enzymes glycogen synthase, which creates glycogen, and phosphorylase, which breaks down glycogen, regulates the metabolism of glycogen.
Phosphorylation inhibits glycogen synthase but activates phosphorylase, indicating a reciprocal regulation between these two enzymes. By activating protein phosphatases and causing a reduction in their phosphorylation, insulin promotes the production of glycogen.
Evolution
All three domains of life have the major metabolic pathways that were present in our last universal common ancestor, including the citric acid cycle and glycolysis. This universal ancestor cell had a significant metabolism of amino acids, nucleotides, carbohydrates, and lipids.
It was prokaryotic and most likely a methanogen. It’s possible that these reactions served as the best remedy for their specific metabolic issues, which is why these ancient pathways persisted throughout later development.
Examples of such pathways include Glycolysis and the citric acid cycle generate their final products quickly and with great efficiency.
Previous metabolic pathways were a part of the ancient RNA world, and the earliest enzyme-based metabolic pathways may have been components of purine nucleotide metabolism.
Numerous theories have been put up to explain the processes via which new metabolic pathways develop.
These include the recruitment of pre-existing enzymes and their assembly into a novel reaction route, the replication and subsequent divergence of whole pathways, and the successive addition of novel enzymes to a short ancestral pathway.
Although the relative significance of these processes is unknown, genetic research has revealed that the enzymes within a route are likely to have a common ancestor, indicating that several pathways have developed gradually.
Step manner using newly developed functions derived from previously completed stages in the process. An alternate theory has been proposed by research that follows the development of protein structures in metabolic networks.
This suggests that enzymes are widely borrowed to carry out comparable tasks in many metabolic pathways. An evolved enzymatic mosaic is produced as a result of these recruiting mechanisms.
A third hypothesis is that certain metabolic components might exist as “modules” that are reusable in other pathways and have comparable effects on various molecules.
Evolution can result in the loss of metabolic activities in addition to the emergence of novel metabolic pathways.
For instance, several parasites lose metabolic pathways that are not necessary for their life and may scavenge the host for preformed amino acids, nucleotides, and carbohydrates. Endosymbiotic species have similar decreased metabolic capacities.
Investigation And Manipulation
Traditionally, a reductionist methodology that concentrates on a particular metabolic route is used to study metabolism.
Utilizing radioactive tracers at the whole-organism, tissue, and cellular levels is very beneficial since it may be used to identify radioactively labeled intermediates and products, therefore defining the pathways from precursors to end products.
The kinetics and inhibitor response of the isolated enzymes that catalyze these chemical processes may then be studied.
Finding the tiny molecules within a cell or tissue is a complementary strategy; the entire collection of these molecules is referred to as the metabolome.
All things considered, these studies provide a clear picture of the composition and operation of basic metabolic pathways, but they fall short when applied to more intricate systems, such as the metabolism of a whole cell.
The picture on the right, which depicts the interactions between just forty metabolites and forty proteins, gives an indication of the intricacy of the metabolic networks in cells, which house thousands of distinct enzymes.
Lists comprising up to 26.500 genes are obtained from the sequences of genomes. But today, with the use of this genetic data, entire networks of biological events may be rebuilt, and more comprehensive mathematical models that might describe and forecast their behavior can be created.
These models are particularly effective when used with the integration of information on gene expression from DNA microarray and proteomics research with pathway and metabolite data gathered using traditional techniques.
These methods have now been used to create a human metabolic model that will direct future biochemical and drug development studies. These models are now employed in network analysis to categorize human illnesses into clusters based on shared metabolites or proteins.
A remarkable example of bow-tie organization is seen in bacterial metabolic networks, which have an architecture that can take in a broad range of nutrients and use a few intermediary common currencies to make a vast array of products and complicated macromolecules.
Metabolic engineering is a key technical application for this knowledge. Here, organisms like yeast, plants, or bacteria are genetically altered to increase their biotechnological use and facilitate the synthesis of pharmaceuticals like antibiotics or industrial compounds like shikimic acid and 1,3-propanediol.
Usually, the goals of these genetic alterations are to enhance yields, decrease waste output, and use less energy to generate the product.
History
Greek Philosophy
The Parts of Animals by Aristotle provides enough information about his theories on metabolism to allow for the creation of an open-flow model.
According to his theory, dietary components underwent a transformation at each step of the process, releasing heat in the form of the classical element of fire and excreting leftover elements such as feces, urine, or bile.
In his work Al-Risalah al-Kamiliyyah fil Siera al-Nabawiyyah, written in 1260 AD, Ibn al-Nafis discussed metabolism.
The body and its constituent parts are inexorably undergoing perpetual change since they are always being dissolved and fed, he asserted.
Implementing Contemporary Metabolic Theories and the Scientific Method
Over the course of several centuries, the scientific study of metabolism has evolved from analyzing complete animals in early investigations to focusing on specific metabolic events in contemporary biochemistry.
Santorio Santorio reported the first controlled tests on human metabolism in his work Ars de static medicine in 1614. He explained that he weighed himself both before and after fasting, drinking, exercising, working, sleeping, and eating.
He discovered that the majority of the food he consumed was lost due to “insensible perspiration”.Living tissue was believed to be animated by a vital force in this early research, as the mechanisms behind these metabolic processes had not yet been discovered.
Louis Pasteur deduced that chemicals found in yeast cells known as “ferments” stimulated the fermentation process when he studied the 19th-century conversion of sugar to alcohol by yeast.
As to his statement, the process of fermenting alcohol is linked to the existence and arrangement of yeast cells, rather than their breakdown or demise.
“Within the cells.” This finding is noteworthy because, in addition to Friedrich Wöhler’s 1828 publication of a paper on the subject, it is the first organic compound composed only of inorganic precursors. the chemical synthesis of urea.
This demonstrated that, in theory, organic molecules and chemical processes present in cells were identical to those found in any other area of chemistry.
The field of biochemistry had its start when Eduard Buchner discovered enzymes around the turn of the 20th century, separating the biological study of cells from the chemical events of metabolism.
During the first part of the 20th century, the body of knowledge on biochemistry expanded quickly. Hans Krebs was one of the most active contemporary biochemists and made significant advances in the field of metabolic research.
Along with Hans Kornberg, he discovered the urea cycle and subsequently the citric acid and glyoxylate cycles. contemporary biochemical New methods including chromatography, and electron microscopy.
Molecular dynamics simulations, X-ray diffraction, NMR spectroscopy, and radioisotopic labeling have tremendously benefited research. These methods have made it possible to identify and thoroughly examine the many chemicals and metabolic processes found in cells.
FAQ
What are the 2 types of metabolism?
Catabolism – the process by which nutrients (such proteins, carbs, and dietary fats) are broken down into their most basic forms so that they may be used as energy sources and the building blocks of growth and repair.
Anabolism – the portion of metabolism that builds and repairs our bodies.
What is the process of the metabolism?
Your body uses a process known as metabolism to convert food and liquids into energy. Your body stores extra energy as fat when it has more than it requires. The idea that certain meals or substances might speed up your metabolism is not well supported by research.
What is an example of metabolism?
The process of making glucose from carbon dioxide is one instance. Other instances include the production of DNA strands from nucleotide building blocks in nucleic acid synthesis or proteins from amino acids.
Does metabolism make you lose fat?
A lot of people attribute weight troubles to metabolic issues. However, your metabolism adjusts on its own to suit your body’s requirements. Rarely does it result in weight gain or reduction. Anyone who burns more calories than they consume will generally lose weight.
What are the three 3 types of metabolism?
Finding out what sort of person you are metabolically will help you tailor your exercise and nutrition to maximize your metabolism. Ectomorph, mesomorph, and endomorph are the three primary metabolic types; they are terms you usually don’t use in day-to-day discussions.
What is the metabolic 2 2 2 method?
Meals categorized as fatty or high in carbohydrates, in Smith’s opinion, belong in the first two groups. The third round of exercises consists of bodyweight and/or high-intensity interval training; the second and third sets include non-food related activities like journaling and weekly weigh-ins.
What are the 4 stages of metabolism?
Science research found that the metabolism goes through four age-related phases: it is very fast in the first year of life, slows down until the age of 20, remains constant between the ages of 20 and 60, and then slows down once again in later life.