ACYANOTIC CONGENITAL HEART DISEASE
Acyanotic heart defects are congenital cardiac malformations that affect the atrial or ventricular walls, heart valves, or large blood vessels. Common causes include genetic defects (e.g., trisomies), maternal infections (e.g., rubella), or maternal consumption of drugs or alcohol during pregnancy. Acyanotic heart defects are characterized pathophysiologically by a left-to-right shunt, which causes pulmonary hypertension and right heart hypertrophy.
The symptoms depend on the extent of the malformation and the resulting impairment of cardiac function. Infants may be asymptomatic or present with exercise intolerance, failure to thrive, and symptoms of heart failure. Characteristic heart murmurs are important clues for establishing the diagnosis, which is typically confirmed by visualizing the defect on echocardiography.
A chest x-ray, MRI, or cardiac catheterization may also be required to determine the indications for surgery and plan the procedure. Acyanotic heart defects requiring treatment are repaired via catheter procedures or surgery. Supportive medical therapy is needed in cases of heart failure (e.g., diuretics, inotropic agents) or if surgery cannot be performed (e.g., prostaglandin). Common complications include arrhythmias, embolisms, and infective endocarditis, especially if treatment is delayed.
Overview of Acyanotic heart defects:
- General clinical features
- Normal skin tone
- Exercise intolerance
- Fatigue
- Exertional tachycardia, pallor, and diaphoresis (sweating)
- Exertional dyspnea, tachypnea
- Recurrent bronchopulmonary infections
- Failure to thrive
- Heart failure in larger defects
- Tachycardia
- Right-sided heart failure
- Hepatic venous congestion with hepatomegaly
- Peripheral edema is rarely seen in infants.
- Left-sided heart failure
- Tachypnea, pulmonary edema
- Low cardiac output: BP, pallor, sweating, cool extremities, poor growth, syncope
Differential cyanosis: cyanosis in the lower extremities when the Eisenmenger reaction occurs
ATRIAL SEPTAL DEFECT (ASD)
EPIDEMIOLOGY
Prevalence: ~ 2/1000 live births
ETIOLOGY of Acyanotic heart defects
Down syndrome
Fetal alcohol syndrome
Holt-Oram syndrome
PATHOPHYSIOLOGY of Acyanotic heart defects
Impaired growth or excessive resorption of the atrial septa in utero leads to atrial septal defects.
Ostium primum atrial septal defect (ASD I): (~ 15–20%)
Ostium secundum atrial septal defect (ASD II): (~ 70%)
Typically a low-pressure, low-volume, minor left-to-right shunt? patients are asymptomatic
In larger defects, the shunt may lead to right heart failure, supraventricular arrhythmias, pulmonary hypertension, cor pulmonale, and/or the Eisenmenger reaction
- CLINICAL FINDINGS
Depend on defect size and shunt volume
Small defects: usually asymptomatic
Large defects - Palpitations
- ASDs typically manifest with advancing age.
- Auscultation
- Systolic ejection murmur over the left second ICS sternal border
- Widely split second heart sound (S2) over the left second ICS, which is fixed (does not change with respiration ), normal S1
- Soft mid-diastolic murmur over the lower left sternal border
DIAGNOSTICS
Echocardiography (confirmatory test): to visualize the defect, its extent, and shunt volume
ECG: vertical or right axis, P pulmonale, right bundle branch block (complete or incomplete), signs of right heart hypertrophy, PR prolongation
Chest x-ray: enlarged right atrium, ventricle, and pulmonary arch; increased pulmonary vasculature
TREATMENT
In childhood, spontaneous closure may occur.
Patch repair
Indicated in symptomatic children with significant left-to-right shunt
Surgical or via percutaneous transcatheter procedure
COMPLICATION
Paradoxical embolism risk of stroke
VENTRICULAR SEPTAL DEFECT (VSD)
EPIDEMIOLOGY
The most common congenital heart defect (4/1000 live births)
Occurs as an isolated heart defect or in combination with others
ETIOLOGY
Genetic syndromes: Down syndrome, Edward syndrome, Patau syndrome
Fetal alcohol syndrome
Intrauterine infection (e.g., TORCH)
PATHOPHYSIOLOGY
Localization: most commonly in the membranous part of the ventricular septum (pars membranacea)
Defect in ventricular septum left-to-right shunt with the following consequences:
LV volume overload left ventricular hypertrophy
Excessive pulmonary blood flow increased pulmonary artery pressures pulmonary hypertension and right-sided heart hypertrophy
Decreased cardiac output
Possibly an Eisenmenger reaction in the late stage of the disease due to irreversible pulmonary hypertension
CLINICAL FINDING
Small defects: usually asymptomatic
Medium-sized or large defects
Lead to cardiac failure in the first 2–3 months of life
Palpation: Hyperdynamic precordium may be detected in hemodynamically relevant defects.
Auscultatory findings
Harsh holosystolic murmur over the left lower sternal border; typically louder in small defects
Mid-diastolic murmur over cardiac apex
Systolic thrill
Loud pulmonic S2 (if pulmonary hypertension develops)
DIAGNOSIS
Doppler echocardiography: confirms diagnosis; evaluation of defect size and shunt volume; exclusion of other anomalies
ECG: signs of right heart hypertrophy
Chest x-ray
Enhanced pulmonary vascular markings
Left atrial and ventricular enlargement
In later stages, enlarged right ventricle and pulmonary artery (due to elevated PVR)
TREATMENT
Small VSDs: rarely require surgical interventions small to moderate defects often heal spontaneously; follow-up echocardiography recommended
Symptomatic and large VSDs
Surgical (patch) repair in children < 1 year of age with signs of pulmonary hypertension and older children who did not improve with medical therapy
Closure of a VSD results in a decrease in right ventricular and left atrial pressures and an increase in left ventricular pressure when compared to pre-treatment values
Heart-lung transplant or lung transplant with concurrent VSD repair if Eisenmenger’s reaction has occurred
- COMPLICATIONS
- Arrhythmias
- Right heart failure
- Eisenmenger’s reaction
- Infective endocarditis
- Aortic regurgitation
CONGENITAL PATENT DUCTUS ARTERIOSUS (PDA)
GLOSSARY
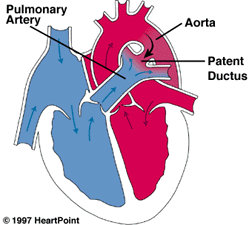
While still in the mother’s womb, a baby does not need their lungs to supply oxygen because they receive oxygen from their mother. Since a baby’s lungs do not provide oxygen, there is no need for the heart to pump blood to the lungs. The ductus arteriosus is a blood vessel that is present in all babies while still in the womb, and it allows blood to bypass the lungs.
When the baby is born and the umbilical cord is cut, the lungs need to supply oxygen to the body. Their lungs expand, their blood vessels relax to accept more blood flow, and the ductus arteriosus usually closes within the first hours of life. Sometimes, the ductus arteriosus does not close on its own. This is known as a patent (“open”) ductus arteriosus. While this condition is seen more often in premature babies, it may also appear in full-term infants.
SYMPTOMS OF PATENT DUCTUS ARTERIOSUS
The symptoms of a patent ductus arteriosus depend on the size of the ductus and how much blood flow it carries. After birth, if a ductus arteriosus is present, blood will flow from the aorta (the main artery in the body) into the pulmonary artery. This extra blood flow into the lungs can overload the lungs and put more burden on the heart to pump this extra blood. Some babies may need more support from a ventilator and have symptoms of congestive heart failure.
A newborn with a patent ductus arteriosus may have:
- Fast breathing
- A hard time breathing
- More respiratory infections
- Tire more easily
- Poor growth
However, if the patent ductus arteriosus is not large, it may cause no symptoms and your doctor may not find it until they do further evaluation of a heart murmur.
Even if there are no symptoms, the turbulent flow of blood through the patent ductus arteriosus puts a person at a higher risk for a serious infection, known as endocarditis.
DIAGNOSIS
Because of turbulent blood flow, a patent ductus arteriosus causes a distinct-sounding heart murmur that is heard on physical exam.
The murmur, along with symptoms of heart failure in a premature infant, most often leads to the diagnosis of patent ductus arteriosus. A chest X-ray will show an enlarged heart and evidence of a large amount of blood flow to the lungs. An echocardiogram is done to confirm the diagnosis. doctor can see the size of the ductus arteriosus and also find out if the heart chambers have become enlarged due to the extra blood flow.
In older children, though, their chest X-ray is typically normal. An echocardiogram will show the flow of blood through the patent ductus arteriosus and is typically done to confirm the diagnosis.
In a newborn, the patent ductus arteriosus still has the chance to close on its own. The doctor may allow more time for the patent ductus arteriosus to close on its own if the heart failure is under control. If a newborn’s symptoms are severe or it is unlikely to close on its own, medical or surgical treatment is needed.
Medicines work best for newborns. They may receive medicine, such as indomethacin or ibuprofen, to constrict the muscle in the wall of the patent ductus arteriosus and help it close. These drugs do have side effects, so not all babies can receive them.
SURGERY
A small incision is made between the ribs on the left side.
The ductus arteriosus is tied and cut.
The risk of complications with any of these treatments is low, determined mostly by how ill the child is prior to treatment.
PDA IS GOOD OR NOT
Yes. Some babies have heart defects that require the patent ductus arteriosus to remain open for them to survive.
In some heart defects, such as pulmonary atresia (an underdeveloped or blocked pulmonary valve), the patent ductus arteriosus supplies the only adequate source of blood flow to the lungs so that oxygen can be delivered to the blood. In these patients, the ductus arteriosus supplies blood to the lungs from the aorta.
COARCTACTION OF AORTA
Coarctation (ko-ahrk-TAY-shun) of the aorta — or aortic coarctation — is a narrowing of the aorta, the large blood vessel that branches off your heart and delivers oxygen-rich blood to body. When this occurs, your heart must pump harder to force blood through the narrowed part of your aorta.
Coarctation of the aorta is generally present at birth (congenital). The condition can range from mild to severe, and might not be detected until adulthood, depending on how much the aorta is narrowed.
Coarctation of the aorta often occurs along with other heart defects. While treatment is usually successful, the condition requires careful lifelong follow-up.
SYMPTOMS
Coarctation of the aorta symptoms depends on the severity of the condition. Most people don’t have symptoms. Children with serious aortic narrowing may show signs and symptoms earlier in life, but mild cases with no symptoms might not be diagnosed until adulthood. People may also have signs or symptoms of other heart defects that they have along with coarctation of the aorta.
- Babies with severe coarctation of the aorta may begin having signs and symptoms shortly after birth. These include:
- Pale skin
- Irritability
- Heavy sweating
- Difficulty breathing
- Difficulty feeding
- Left untreated, aortic coarctation in babies might lead to heart failure or death.
Older children and adults with coarctation of the aorta often don’t have symptoms because their narrowing may be less severe. If you have signs or symptoms that appear after infancy, you most commonly will have high blood pressure (hypertension) measured in your arms. However, your blood pressure is likely to be lower in your legs.
- SIGNS AND SYMPTOMS
- High blood pressure
- Headache
- Muscle weakness
- Leg cramps or cold feet
- Nosebleeds
- Chest pain
Seek medical help if the child has the following signs or symptoms:
- Severe chest pain
- Fainting
- Sudden shortness of breath
- Unexplained high blood pressure
- While experiencing these signs or symptoms doesn’t necessarily mean that you have a serious problem, it’s best to get checked out quickly. Early detection and treatment might help save your life.
CAUSES
Rarely, coarctation of the aorta develops later in life. Traumatic injury might lead to coarctation of the aorta. Rarely, severe hardening of the arteries (atherosclerosis) or a condition causing inflamed arteries (Takayasu’s arteritis) can narrow the aorta, leading to aortic coarctation.
Coarctation of the aorta usually occurs beyond the blood vessels that branch off to your upper body and before the blood vessels that lead to your lower body. This can often lead to high blood pressure in your arms but low blood pressure in your legs and ankles.
With coarctation of the aorta, the lower left heart chamber (left ventricle) of your heart works harder to pump blood through the narrowed aorta, and blood pressure increases in the left ventricle. This may cause the wall of the left ventricle to thicken (hypertrophy).
Prevention
Coarctation of the aorta can’t be prevented, because it’s usually present at birth (congenital). However, if you or your child has a condition that increases the risk of aortic coarctation, such as Turner syndrome, bicuspid aortic valve, or another heart defect, or a family history of congenital heart disease, early detection can help. Discuss the risk of aortic coarctation with your doctor.




One Comment