Oxygen
Introduction
The chemical element oxygen has the atomic number eight and the symbol O. It is a highly reactive nonmetal that belongs to the chalcogen group in the periodic table.
It is also an oxidizing agent that easily produces oxides with most elements and other compounds. The most prevalent element in the crust of the Earth, oxygen is the third most plentiful element in the universe, after helium and hydrogen.
With eight protons in its nucleus, oxygen is an atomic number eight chemical element. At normal temperatures and pressures, oxygen forms the colorless gas O2, a molecule made up of two atoms. There are four ways that chemists depict molecular oxygen. Conventionally, oxygen is shown as red in the colored models.
At normal oxygen, a colorless and odorless diatomic gas with the formula O2 is created when two atoms of the element join together at a certain temperature and pressure.
The Earth’s atmosphere is now made up of 20.95% diatomic oxygen gas, however, this has fluctuated significantly over a very long time. Oxygen is found in almost half of the Earth’s crust in the form of oxides.
For cellular respiration, which derives energy from food molecules by reacting oxygen with them and generates carbon dioxide as waste, all plants, animals, and fungi require oxygen.
Breathing allows carbon dioxide to flow out of the circulation and oxygen to diffuse into the blood in tetrapods, where gas exchange occurs in the lungs. The oxygen is delivered to the cells by the circulatory system of the body.
Where it is used for cellular respiration. Just as the main component inorganic compounds of animal shells, teeth, and bone include oxygen atoms, so do many key types of organic molecules found in living beings, such as proteins, nucleic acids, carbohydrates, and lipids.
Water, the primary component of lifeforms, contains oxygen, which makes up the majority of the mass of living things.
Photosynthesis is the process by which oxygen is continually produced from carbon dioxide and water using solar energy, replenishing the oxygen in the atmosphere of the Earth.
Because of its high chemical reactivity, oxygen cannot exist in the air as a free element without being constantly replaced by the process of photosynthetic organisms. Ozone (O3), another type of oxygen, is an allotrope that absorbs ultraviolet B light very well.
The high-altitude ozone layer shields the biosphere from UV radiation. On the other hand, ozone near the surface is a pollutant as it is a consequence of smog.
Although Michael Sendivogius isolated oxygen before 1604, it is generally accepted that Joseph Priestley in Wiltshire in 1774 and Carl Wilhelm Scheele in Uppsala in 1773 or earlier made their separate discoveries of the element.
Priestley’s work is frequently given precedence because it was published first. However, Priestley did not acknowledge oxygen as a chemical element and instead referred to it as “dephlogisticated air”.
Antoine Lavoisier, who first identified oxygen as a chemical element and accurately described its function in combustion, is credited with coining the word oxygen in 1777.
The manufacture of steel, plastics, and textiles; brazing; welding; cutting of steel and other metals; rocket propellant; oxygen therapy; and life support systems in spaceflight, diving, submarines, and airplanes are among the common applications of oxygen.
History of Study
Early Experiments
Greek mechanic Philo of Byzantium carried out one of the earliest documented tests on the connection between air and combustion in the second century BCE.
Philo noted in his work Pneumatica that when a vessel was turned over on a burning candle and water was poured around its neck, part of the water rose up into the neck.
Philo made the mistake of supposing that some of the air inside the vessel had been changed into the classical element fire, which allowed it to escape through the glass’s pores. Several centuries after Philo’s discovery.
Leonardo da Vinci expanded on it when he noted that respiration and burning both use some air. Robert Boyle established that burning requires air in the late 17th century.
John Mayow (1641–1679), an English chemist, improved this work by demonstrating that fire needs only a certain portion of air, which he named spiritus nitroaereus.
In one experiment, he discovered that submerging a lighted candle or a mouse in a closed container over water led the water to rise and replace a quarter of the volume of the air before the creatures were put out of their misery.
Based on this, he deduced that both respiration and combustion need nitroaereus. Mayow deduced that the nitroaereus must have amalgamated with antimony since he saw that antimony gained weight when heated.
Additionally, he believed that nitroaereus reacts with particular molecules in the body to produce heat and muscular activity in animals and that the lungs extract nitroaereus from the air and transfer it into the blood.
In his book Tractatus Duo, he provided accounts of these and other trials and concepts in the tract “De respiratione” in 1668.
Phlogiston Theory
In the 17th and 18th centuries, oxygen was generated in experiments by Mikhail Lomonosov, Pierre Bayen, Robert Hooke, and Ole Borch, but none of them identified oxygen as a chemical element.
The phlogiston hypothesis, which at the time was the preferred explanation for those processes, may have contributed to this in part because of its widespread use in the philosophy of corrosion and combustion.
Phlogiston theory was developed in 1667 by the German alchemist J. J. Becher and revised in 1731 by the chemist Georg Ernst Stahl. It proposed that all flammable materials were composed of two components.
When the substance holding it burnt, one portion, known as phlogiston, was released; the dephlogisticated part was believed to be its actual form or calx.
Phlogiston was believed to make up most of highly flammable, low-residue materials like coal or wood, and very little of non-combustible, corrosive elements like iron.
Phlogiston’s hypothesis was founded on observations of what occurs when something burns, that is, most ordinary items look to get lighter and seem to lose something in the process. Neither air nor the first quantitative studies designed to prove the theory were involved.
Discovery
Michael Sendivogius, a Polish physician, philosopher, and alchemist, wrote: “Twelve Treatises on the Philosopher’s Stone Drawn from the Source of Nature and Manual Experience” (De Lapide Philosophorum Tractatus duodecim e naturae fonte et manual experiential deposit).
(1604) named a material found in air as “cibus vitae,” or “food of life.” Polish historian Roman Bugaj claims that this material is the same as oxygen.
Sendivogius correctly identified the material as the gaseous byproduct that is generated during the heat breakdown of potassium nitrate during his studies conducted between 1598 and 1604.
According to Bugaj, there is enough proof that Sendivogius discovered oxygen because of the isolation of the gas and the appropriate correlation of the material with the area of the air that is necessary for life.
However, the generations of scientists and chemists that came after Sendivogius disregarded his discoveries. It’s also a popular belief that Swedish chemist Carl Wilhelm Scheele made the initial discovery of oxygen.
In 1771–1772, he heated mercuric oxide (HgO) and other nitrates to make oxygen gas. At the time, Scheele dubbed the gas “fire air” as it was the only known substance that could facilitate burning in 1775.
He submitted to his publisher a manuscript titled Treatise on Air and Fire, which contained a description of this finding. In 1777, that document was published. Meanwhile, on August 1, 1774,
British cleric Joseph Priestley conducted an experiment in which he exposed mercuric oxide in a glass tube to sunlight, resulting in the release of a gas he called “dephlogisticated air”.
He saw that the gas caused candles to burn brighter and that breathing it made mice more active and longer-lived. Priestley stated, “The feeling of it to my lungs was not sensibly different from that of common air,” but he had experienced the gas firsthand.
“For a while afterward, I had the strangest sensation of my breasts being light and easy.” The second volume of Priestley’s book, Experiments, contains the findings he presented in 1775. and Observations on Different Kinds of Air, a piece titled “.
An Account of Further Discoveries in Air”. Priority in the discovery is typically accorded to Priestley since he reported his results first.
Subsequently, the new compound was reported to have been independently discovered by the French scientist Antoine Laurent Lavoisier.
In October 1774, Priestley went to see Lavoisier and informed him about his experiment and the process by which he had freed the new gas.
Additionally, on September 30, 1774, Scheele sent Lavoisier a letter outlining his finding of the hitherto unidentified material; however, Lavoisier never acknowledged receiving it. (A copy of the letter was discovered in Scheele’s posthumous possessions.)
Lavoisier’s Contribution
Lavoisier provided the first accurate description of how combustion occurs and carried out the first sufficient quantitative studies on oxidation.
He employed these and related tests, which he began in 1774, to refute the phlogiston theory and demonstrate that the material that Priestley and Scheele had found was a chemical element.
In one experiment, Lavoisier found that heating tin and air in a closed container did not result in an overall increase in weight. When he opened the container, he saw that air shot in, indicating that some of the stored air had been used up.
He also observed that the weight gain in the tin was equal to the weight of the air that rushed back in. His book Sur la Combustion included documentation of these and other tests. Publication en général, which came out in 1777.
In that study, he demonstrated that air is a combination of two gases: azote, “lifeless”), which could not support respiration or combustion, and “vital air,” which is necessary for both. Although it still goes by the previous name in French and a few other European languages, azote was eventually renamed nitrogen in English.
Etymology
Erroneously believing that oxygen was a part of all acids, Lavoisier changed “vital air” to oxygène in 1777, drawing on the Greek words (oxy) (acid, literally “sharp”, from the taste of acids) and (-genes) (producer, meaning begetter).
Lavoisier was mistaken in this regard, as chemists (including Sir Humphry Davy in 1812) would later discover, but by then the name had become too well-known.
Despite objections from English scientists and the fact that the gas was initially isolated and described by an Englishman, Priestley, oxygen entered the English language.
In Erasmus Darwin’s well-known book The Botanic Garden (1791), Charles Darwin’s grandpa, there is a poem named “Oxygen” that praises the gas.
Later History
According to John Dalton’s initial atomic hypothesis, all elements were monatomic, and compounds’ atoms would typically have the simplest atomic ratios among themselves.
For instance, Dalton concluded that the formula for water was HO, which meant that the atomic mass of oxygen was eight times that of hydrogen, rather than the more accurate number of about 16.
Water is made up of two volumes of hydrogen and one volume of oxygen, as demonstrated by Joseph Louis Gay-Lussac and Alexander von Humboldt in 1805.
By 1811, Amedeo Avogadro had determined the correct composition of water by using the diatomic elemental molecules in those gases and what is now known as Avogadro’s law.
The so-called Brin process, which used a reversible reaction of barium oxide, was the first commercial technique for manufacturing oxygen.
It was developed in 1852 and brought to market in 1884, but in the early 20th century, newer techniques took its place. By the late 1800s, scientists had discovered that by compressing and chilling air, it could be liquefied and its constituent parts separated.
Swiss scientist and chemist Raoul Pierre Pictet used a cascade mechanism to evaporate liquid sulfur dioxide, which liquefied carbon dioxide, which liquefied oxygen gas by cooling it down enough.
On December 22, 1877, he announced his discovery of liquid oxygen by telegram to the French Academy of Sciences in Paris. French scientist Louis Paul Cailletet said just two days later his unique technique for melting molecular oxygen.
In each case, only a few drops of the liquid were created, making substantial analysis impossible. On March 29, 1883, Polish physicists Zygmunt Wróblewski and Karol Olszewski of Jagiellonian University liquefied oxygen for the first time in a stable condition.
James Dewar, a Scottish scientist, succeeded in producing enough liquid oxygen for research in 1891. German engineer Carl von Linde and British engineer William Hampson separately invented the first economically feasible method for manufacturing liquid oxygen in 1895.
The air was cooled until it liquefied, and both men separated the constituent gases by boiling them off one at a time and catching them individually.
Eventually, in 1901, a combination of compressed oxygen and acetylene was burned to show oxyacetylene welding for the first time. Later on, this technique for cutting and welding metal became widespread.
The first liquid-fuel rocket engine was created in 1923 by American scientist Robert H. Goddard; it ran on gasoline as the fuel and liquid oxygen as the oxidant.
On March 16, 1926, Goddard successfully launched a 56-meter liquid-fueled rocket near Auburn, Massachusetts, reaching 97 kilometers per hour. Potassium chlorate combined with a small amount of manganese dioxide may be heated in academic labs to produce oxygen.
Globally, atmospheric oxygen levels are slightly declining, presumably as a result of burning fossil fuels.
Characteristics
Properties and Molecular Structure
Oxygen, sometimes known as dioxygen, is a colorless, odorless, and tasteless gas having the chemical formula O2 at ordinary temperature and pressure. Two oxygen atoms are chemically bonded to one another to form dioxygen.
Depending on the theoretical level, the bond may be characterized in a variety of ways, but the most straightforward explanation is that it is a covalent double bond that is created when atomic orbitals of individual oxygen atoms fill molecular orbitals, giving the bond an order of two.
More precisely, the Aufbau, or sequential filling of orbitals from low to high energy, is what creates the double bond, and the cancellation of contributions from the 2s the two atomic 2p orbitals that lie along.
The O-O molecular axis and the two pairs of atomic 2p orbitals perpendicular to it, followed by the sequential filling of the low s and s* orbitals; the contributions from the remaining two 2p electrons are then canceled after their partial filling of the p* orbitals.
Dioxygen exhibits a triplet electronic ground state along with its double-bond nature and reactivity due to a combination of cancellations and s and p overlaps.
A spin triplet state is an electron configuration containing two unpaired electrons, such as those found in dioxygen orbitals (note the filled p* orbitals in the picture) that are degenerate, or of equal energy.
As a result, triplet oxygen refers to the ground state of the O2 molecule. Filling the highest-energy, partly-filled orbitals reduces the bond order from three to two since they are antibonding.
Spontaneous combustion is prevented by triplet oxygen’s unpaired electrons, which react relatively slowly with most organic compounds that have paired electron spins. The O2 molecules are paramagnetic in the triplet state.
In other words, because of the spin magnetic moments of the unpaired electrons in the molecule and the negative exchange energy between nearby O2 molecules, they provide oxygen magnetic character given the existence of a magnetic field.
Because liquid oxygen is highly magnetic, it may be used to maintain a liquid oxygen bridge against its own weight between the poles of a strong magnet in laboratory experiments. Several higher-energy forms of molecular O2 with paired electron spins are referred to be singlet oxygen.
When it comes to typical organic compounds, it is far more reactive than regular (triplet) molecular oxygen. In nature, photosynthesis, which uses solar energy, frequently produces singlet oxygen from water.
In the troposphere, it is also generated by the immune system as a source of active oxygen and by the photolysis of ozone by short-wavelength light.
Before singlet oxygen may damage tissues, carotenoids in photosynthetic organisms (and maybe mammals) are essential in absorbing the energy and transferring it to the unexcited ground state.
Allotropes
Dioxygen, or O2, is the prevalent allotrope of elemental oxygen found on Earth and makes up the majority of the atmosphere. O2 has a bond energy of 498 kJ/mol and a bond length of 121 pm.
Complex living forms, including mammals, utilize oxygen for cellular respiration. The rest of this article discusses other facets of O2.
Trioxygen (O3), sometimes referred to as ozone, is a very reactive oxygen allotrope that is harmful to lung tissue. As O2 splits by ultraviolet (UV) light, atomic oxygen is created in the upper atmosphere, where ozone is created.
The earth is shielded from radiation by the ozone layer in the upper atmosphere because ozone absorbs light in the ultraviolet spectrum. It is a pollutant that is produced as a byproduct of vehicle emissions close to the surface of the Earth.
There is enough atomic oxygen available at low earth orbit altitudes to induce spacecraft corrosion. After its discovery in 2001, the metastable molecule tetraoxygen (O4) was thought to be present in one of the six phases of solid oxygen.
This phase, which was produced by pressurizing O2 to 20 GPa, was shown to be a rhombohedral O 8 cluster in 2006. This cluster might be utilized as rocket fuel since it has the potential to be a far stronger oxidant than either O2 or O3.
When solid oxygen is exposed to pressures more than 96 GPa, a metallic phase was found in 1990. It was demonstrated in 1998 that this phase becomes superconducting at very low temperatures.
Physical Properties
In freshwater, oxygen dissolves more easily than in saltwater, and it dissolves in water more easily than nitrogen does. When water and air are in balance, there is around one dissolved oxygen molecule for every two N2 molecules (1:2), but the atmospheric ratio is closer to 1:4.
| 5 °C | 25 °C | |
| Freshwater | 9.00 | 6.04 |
| Seawater | 7.20 | 4.95 |
(milliliters per liter)
Temperature affects how soluble oxygen is in water; at 0 °C, 14.6 mg/L dissolves more readily than at 20 °C, where 7.6 mg/L does. 25 °C and In one normal atmosphere (101.3 kPa) of air, 6.04 milliliters (mL) of oxygen can dissolve in one liter of fresh water.
Whereas 4.95 mL of oxygen can dissolve in one liter of seawater. The solubility rises to 7.2 mL (45% more) per liter for seawater and 9.0 mL (50% more) per liter for freshwater at 5 °C. At 90.20 K (-182.95 °C, -297.31 °F), oxygen condenses, and at 54.36 K (-218.79 °C, -361.82 °F), it freezes.
O2 is a transparent material that is both liquid and solid. Its light sky-blue hue is a result of absorption in the red, as opposed to the sky’s blue color, which is the result of blue light being scattered by Rayleigh.
Liquefied air is often subjected to fractional distillation to produce high-purity liquid oxygen. Using liquid nitrogen as a coolant, liquid oxygen may also be condensed from air. Since liquid oxygen is extremely reactive, it needs to be kept apart from flammable objects.
Molecular oxygen spectroscopy is linked to airglow and aurora atmospheric processes. Atomic oxygen is produced by absorption in the ultraviolet Schumann-Runge and Herzberg continuum bands, and this oxygen is crucial to the middle atmosphere’s chemistry. Red chemiluminescence in solution is caused by excited-state singlet molecular oxygen.
Table of thermal and physical properties of oxygen (O2) at atmospheric pressure:
| Temperature (K) | Density (kg/m^3) | Specific heat (kJ/kg °C) | Dynamic viscosity (kg/m s) | Kinematic viscosity (m^2/s) | Thermal conductivity (W/m °C) | Thermal diffusivity (m^2/s) | Prandtl Number |
Isotopes and Stellar Origin
Three stable isotopes make up naturally occurring oxygen: 16O, 17O, and 18O. Of them, 16O has the highest natural abundance (99.762%). While some 16O is created during the burning of neon, the majority is created in big stars at the conclusion of the helium fusion process.
17O is a prevalent isotope in the hydrogen-burning zones of stars because it is mostly produced during the CNO cycle when hydrogen is burned to produce helium.
The majority of 18O is created when 14N, which is plentiful due to CNO burning, absorbs a 4He nucleus, which is why 18O is frequently found in the helium-rich regions of developed, massive stars.
The range of described radioisotopes is 11O to 28O, with a total of fifteen. With half-lives of 122.24 seconds for 15O and 70.606 seconds for 14O, respectively, they are the most stable materials.
There are still radioactive isotopes with half-lives of less than 27 seconds for all of them and less than 83 milliseconds for most of them.
When isotopes less than 16O decay, they most often produce nitrogen through ß+ decay, while when they decay larger than 18O, they most often produce fluorine through beta decay.
Occurrence
In terms of mass, oxygen is the most prevalent chemical element in the biosphere, air, sea, and land on Earth. After helium and hydrogen, oxygen is the third most common chemical element in the universe.
Oxygen makes up around 0.9% of the Sun’s mass. As part of oxide compounds like silicon dioxide, oxygen makes up 49.2% of the Earth’s crust by mass, making it the most prevalent element in the crust relative to mass.
Additionally, it makes up the majority of the world’s seas (88.8% of their mass). The oxygen gas that is most frequently found in the atmosphere of Earth, makes up around 1015 tonnes or 23.1% of its mass and 20.8% of its volume.
With a concentration of oxygen gas in its atmosphere of 0.1% by volume, Mars and Venus have far lower levels of oxygen gas than Earth, making Earth unique among the planets in the Solar System.
The only process that produces the O2 around those planets is the interaction of UV light with molecules that contain oxygen, such as carbon dioxide. The oxygen cycle is the cause of Earth’s abnormally high concentration of oxygen gas.
The flow of oxygen within and between its three primary reservoirs on Earth—the atmosphere, the biosphere, and the lithosphere—is described by this biogeochemical cycle.
Photosynthesis is the primary process that powers the oxygen cycle and creates the atmosphere of the modern Earth.
| Z | Element | Mass fraction in parts per million | Mass fraction in parts per million |
| 1 | Hydrogen | 739,000 | 71 × mass of oxygen (red bar) |
| 2 | Helium | 240,000 | 23 × mass of oxygen (red bar) |
| 6 | Carbon | 4,600 | |
| 7 | Nitrogen | 960 | |
| 8 | Oxygen | 10,400 | |
| 10 | Neon | 1,340 | |
| 12 | Magnesium | 580 | |
| 14 | Silicon | 650 | |
| 16 | Sulfur | 440 | |
| 26 | Iron | 1090 |
Oxygen is released into the atmosphere by photosynthesis and removed from it by respiration, decomposition, and combustion. Production and consumption are occurring at the same rate in the current equilibrium.
The world’s aquatic bodies also contain free oxygen in solution. Ocean life is significantly impacted by O2‘s greater solubility at lower temperatures (see Physical characteristics), as polar oceans have higher oxygen contents and hence sustain a far higher density of life.
The process of eutrophication occurs when water contaminated with plant nutrients, such as phosphates or nitrates, encourages the growth of algae. As these organisms and other biomaterials decompose, the O2 level of eutrophic water bodies may decrease.
By calculating the water’s biochemical oxygen demand, or the quantity of O2 required to get it back to a normal concentration, scientists can evaluate this component of water quality.
Analysis
In order to ascertain the climate millions of years ago, paleoclimatologists examine the ratio of oxygen-18 to oxygen-16 in the bones and shells of marine creatures (see oxygen isotope ratio cycle).
At lower temperatures, seawater molecules holding the lighter isotope, oxygen-16, evaporate somewhat more quickly than water molecules containing the 12% heavier isotope, oxygen-18.
There seems to be more snow and rain from that evaporated water at times when global temperatures are lower.in oxygen-16, whereas the residual saltwater has a tendency to contain more oxygen-18.
Then, in comparison to what they would in a warmer environment, marine species integrate more oxygen-18 into their shells and skeletons.
This ratio may also be measured directly by paleoclimatologists in the water molecules of ice core samples that date back hundreds of thousands of years.
Since the Sun’s isotope ratios are thought to be the same as those of the early solar nebula, planetary geologists have studied the relative amounts of oxygen isotopes in samples from meteorites, the Earth, the Moon, and Mars.
However, they have long been unable to establish reference values for these ratios. A silicon wafer that was retrieved from the wrecked Genesis spacecraft and exposed to the solar wind in space has been analyzed.
The results indicate that the Sun contains a larger concentration of oxygen-16 than the Earth. The finding suggests that before the dust grains coalesced to become the Earth, an unidentified activity removed oxygen-16 from the Sun’s disk of protoplanetary material.
Two spectrophotometric absorption bands are visible in oxygen, with peaks at 687 and 760 nm in wavelength.
Certain experts who study remote sensing have suggested that plant health status can be determined from a satellite platform by measuring the light emitted by vegetation canopies in particular bands.
This method makes use of the ability to distinguish between the vegetation’s much lower fluorescence and reflectance in those wavelengths.
Due to the physical structure of plants and the poor signal-to-noise ratio, the measurement is technically challenging; yet, it has been suggested as a potential way to monitor the carbon cycle globally from satellites.
Biological Production and Role of O2
Photosynthesis and Respiration
In the natural world, light-induced water splitting during oxygenic photosynthesis produces free oxygen.
Roughly 70% of Earth’s free oxygen is thought to be created by green algae and cyanobacteria in maritime conditions, with terrestrial plants producing the remaining 30%.
A comparison of studies shows that the seas contribute around 45% of Earth’s atmospheric oxygen annually, with some estimates being lower and some higher.
A streamlined general equation for photosynthesis is :
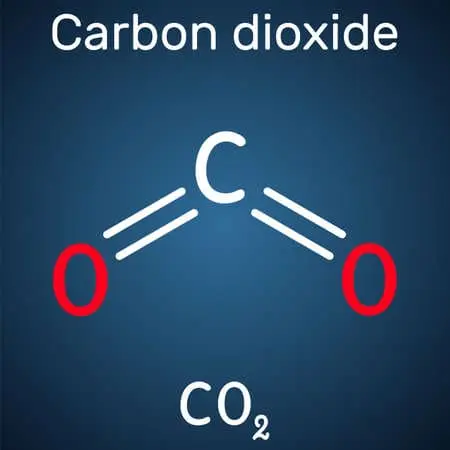
6 CO2 + 6 H2O + photons → C6H12O6 + 6 O2
or just
carbon dioxide + water + sunlight → glucose + dioxygen
Four photons of energy are needed for photolytic oxygen evolution, which takes place in the thylakoid membranes of photosynthetic organisms.
After a number of processes, a proton gradient is created across the thylakoid membrane and utilized to initiate the photophosphorylation process, which produces adenosine triphosphate (ATP).
Following the synthesis of the water molecule, the leftover O2 is released into the environment. In mitochondria, oxygen is used during oxidative phosphorylation to produce ATP. Aerobic respiration’s reaction is essentially photosynthesis’ opposite and is expressed as
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + 2880 kJ/mol
O2 diffuses into red blood cells in vertebrates through lung membranes. When hemoglobin binds oxygen, its color changes from blue-red to brilliant red (the Bohr effect releases CO2 from another area of hemoglobin).
Hemocyanin is used by some arthropods and mollusks, whereas hemerythrin is used by spiders and lobsters. 200 cm3 of O2 may dissolve in one liter of blood. Prior to the identification of anaerobic metazoa, it was believed that all sophisticated life required oxygen.
Reactive byproducts of oxygen consumption in organisms include superoxide ion (O2) and hydrogen peroxide (H2O2).
Higher creatures’ immune systems produce singlet oxygen, superoxide, and peroxide to eliminate invasive microorganisms. In addition, reactive oxygen species are crucial for plants’ hypersensitive defense against pathogen invasion.
Obligately anaerobic species, which constituted the majority of early life on Earth until oxygen started to build up in the atmosphere during the Great Oxygenation Event around 2.5 billion years ago, a billion years after these organisms initially appeared, are harmed by oxygen.
A resting adult takes in between 1.8 and 2.4 grams of oxygen every minute. This translates to about 6 billion tons of oxygen being breathed by people annually.
Living Organisms
The unbound oxygen A living vertebrate organism’s partial pressure is highest in its respiratory system and falls in its peripheral tissues, venous system, and any artery system, in that order. The pressure that oxygen would have on its own if it filled the volume is known as partial pressure.
| Unit | Alveolar pulmonary gas pressures | Arterial blood oxygen | Venous blood gas |
| kPa | 14.2 | 11[h]-13[h] | 4.0[h]-5.3[h] |
| mmHg | 107 | 75[83]-100[83] | 30[84]-40[84] |
Build-up in The Atmosphere
The Earth’s atmosphere had virtually little free oxygen gas until photosynthetic bacteria and archaea developed, most likely 3.5 billion years ago.
Between 3.0 and 2.3 billion years ago, during the Paleoproterozoic epoch, free oxygen first became noticeable.
The banded iron formations appear to have been formed by anoxygenic or micro-aerophilic iron-oxidizing bacteria that dominated the deeper areas of the photic zone while oxygen-producing cyanobacteria covered the shallows.
Even though there was a significant amount of dissolved iron in the oceans when oxygenic photosynthesis was becoming more common. 3–2.7 billion years ago was when free oxygen started to escape from the seas, and it peaked at 10% of its current concentration about 1.7 billion years ago.
The majority of the existing anaerobic species may have gone extinct during the Great Oxygenation Event (oxygen disaster) that occurred around 2.4 billion years ago due to the abundance of free and dissolved oxygen in the seas and atmosphere.
Aerobic organisms may create a lot more ATP than anaerobic species because they use oxygen for cellular respiration. All eukaryotes, or complex multicellular creatures like plants and animals, breathe oxygen through their cells.
There has been a range of 15% to 30% by volume in the atmospheric O2 levels since the Cambrian epoch began 540 million years ago.
The huge size of insects and amphibians during this period may have been influenced by atmospheric O2 levels that peaked at 35% by volume towards the end of the Carboniferous epoch (about 300 million years ago).
Previous climates have been influenced by variations in the amount of oxygen in the air. As air density decreased due to a decrease in oxygen, surface evaporation rose, leading to an increase in precipitation and a rise in temperature.
Regenerating all of the O2 in the current atmosphere would take around 2,000 years at the current pace of photosynthesis. Earth’s oxygen supply is predicted to persist for around a billion years.
Extraterrestrial Free Oxygen
Oxygen is a potent biosignature in the study of astrobiology and the hunt for alien life. However, given that it may have been created abiotically on celestial worlds with unique hydrospheres and other mechanisms that permit free oxygen, such as the thin oxygen atmospheres on Europa and Ganymede, it may not be a definitive biosignature.
Industrial Production
Every year, two main techniques are used to harvest 100 million tons of oxygen from the air for industrial purposes. Fractional distillation of liquefied air is the most often used technique; O2 remains a liquid while N2 distills as a vapor.
One bed of two identical zeolite molecular sieves is passed through with a stream of clean, dry air to produce O2 by absorbing nitrogen and creating a gas stream that is 90% to 93% O2.
This is the second main technique of manufacturing O2. By lowering the working pressure in the chamber and directing some of the oxygen gas from the production bed through it in the opposite direction of flow, nitrogen gas is simultaneously released from the other nitrogen-saturated zeolite bed.
Gaseous oxygen may be pushed continuously through a pipeline because the two beds operate in opposite ways after a predetermined cycle period. Pressure swing adsorption is the term used for this.
These non-cryogenic methods are increasingly being used to generate oxygen gas (see also the related vacuum swing adsorption).
Water may also be electrolyzed to form hydrogen and oxygen molecules, which is another way to create oxygen gas. It is necessary to employ DC power; if AC is utilized, the explosive ratio of oxygen to hydrogen is present in each limb’s gas.
The electrocatalytic O2 evolution from oxides and oxoacids is a technique that is comparable. Additionally, chemical catalysts may be utilized in systems like chemical oxygen generators and oxygen candles.
Which are a part of underwater life support systems. are still a typical feature on commercial aircraft in the event that depressurization occurs.
In order to obtain practically pure O2 gas, another technique for air separation involves applying high pressure or an electric current to force air to dissolve through zirconium dioxide-based ceramic membranes.
Storage
Chemical compounds, cryogenics, and high-pressure oxygen tanks are examples of oxygen storage techniques.
Because liquefied oxygen is cheaper than gaseous oxygen at atmospheric pressure and 20 °C (68 °F), it is frequently carried in large quantities as a liquid in specially insulated tankers.
These tankers are used to replenish bulk liquid oxygen storage tanks that are situated outside of medical facilities and other establishments that require significant amounts of pure oxygen gas.
Heat exchangers are used to transform the cryogenic liquid into gas before the liquid oxygen enters the building. Additionally, oxygen is transported and stored in smaller cylinders that hold compressed gas; these cylinders can be used for oxy-fuel welding and cutting as well as other portable medical uses.
Applications
Medical
Since breathing depends on absorbing oxygen from the air, oxygen supplementation is a common practice in medicine.
In addition to raising the patient’s blood oxygen levels, the treatment also has the unintended benefit of lowering blood flow resistance in many different kinds of lung diseases, which lessens the strain on the heart.
Emphysema, pneumonia, congestive heart failure, some illnesses causing elevated pulmonary artery pressure, and any illness affecting the body’s capacity to absorb and utilize gaseous oxygen are all treated with oxygen treatment.
The treatments are adaptable enough to be utilized by patients at home, in hospitals, and increasingly on portable devices.
Although oxygen tents were originally widely used for oxygen supplementation, oxygen masks, and nasal cannulas have largely taken the place of oxygen tents in recent years.
Special oxygen chambers are used in hyperbaric (high-pressure) medicine to raise the partial pressure of oxygen around the patient and the medical personnel when necessary.
This technique is often used to treat decompression sickness (the “bends”), gas gangrene, and carbon monoxide overdose. The lungs’ increased O2 concentration aids in removing carbon monoxide from hemoglobin’s heme group.
The anaerobic bacteria that cause gas gangrene are poisoned by oxygen gas, therefore raising its partial pressure aids in their death.
Divers that decompress can get decompression sickness. too soon after a dive, causing inert gas bubbles to develop in the blood, primarily nitrogen and helium.
In order for these extra gases to be naturally expelled through the lungs, it is helpful to redissolve the bubbles back into the blood as quickly as possible by raising the pressure of oxygen.
For any diving accident where inert gas bubble formation may occur in the tissues, normobaric oxygen injection at the maximum concentration commonly serves as first aid. Its application is supported epidemiologically by a statistical analysis of instances found in a long-term database.
Life Support and Recreational use
Modern space suits use oxygen dioxide (O2) as a low-pressure breathing gas, enveloping the wearer’s body in the gas. These devices provide a normal blood partial pressure of O2 by using almost pure oxygen at a pressure of roughly one-third that of normal.
To retain suitable flexibility, a higher oxygen concentration must be traded for a lower pressure. Artificially supplied O2 is also necessary for underwater divers and submariners who are supplied via scuba and the surface.
Normal atmosphere pressure is the operating pressure for atmospheric diving suits, submersibles, and submarines. To maintain a steady partial pressure, carbon dioxide in breathing air is removed chemically and replaced with oxygen.
Air or gas mixes with an oxygen proportion appropriate for the operating depth are breathed by ambient pressure divers.
When diving at pressures greater than atmospheric, the use of pure or almost pure oxygen is typically restricted to order to treat acute oxygen poisoning without the risk of drowning, rebreathers, decompression at relatively shallow depths (~6 meters or less), or medical therapy in compression.
Chambers at pressures up to 2.8 bar is recommended. To avoid oxygen toxicity during deeper diving, a considerable dilution of oxygen (O2) with other gases, such as nitrogen or helium, is necessary.
Individuals who travel in non-pressurized fixed-wing airplanes or climb mountains occasionally carry extra oxygen supply. When the cabin depressurizes, pressurized commercial aircraft automatically provide the passengers with an emergency supply of oxygen.
Oxygen masks fall when chemical oxygen generators above each seat are activated by a sudden reduction in cabin pressure.
As directed by the cabin safety regulations, pulling on the masks “to start the flow of oxygen” introduces iron filings into the sodium chlorate within the canister. The exothermic process then produces a continuous stream of oxygen gas.
Due to its moderate euphoric properties, oxygen has long been used recreationally in sports and oxygen bars. Since the late 1990s, there have been venues in the US known as “oxygen bars,” which charge a small price for exposure to oxygen that is higher than usual.
In order to improve performance, professional athletes—particularly in American football—will occasionally take off the field in between plays to put on oxygen masks. It is more likely that there was a placebo effect than a pharmaceutical impact.
Studies that are now available only support an oxygen-enriched mixture’s ability to improve performance when breathed during aerobic activity.
Pyrotechnic uses, like George Goble’s five-second BBQ grill igniting, are other recreational uses that don’t need breathing.
Industrial
The process of smelting iron ore into steel uses up to 55% of oxygen generated for commercial use. Through the use of a high-pressure lance, O2 is pumped into molten iron in this process, eliminating excess carbon and sulfur impurities as the corresponding oxides, SO2, and CO2.
Because of the exothermic processes, the temperature rises to 1,700 °C. The chemical sector consumes an additional 25% of oxygen generated for commercial purposes.
When ethylene and oxygen combine to form ethylene oxide, ethylene glycol is produced. Ethylene is the main raw material needed to make ethylene glycol, which is then used to make a variety of goods.
Such as polyester polymers (which are the building blocks of many plastics and textiles) and antifreeze. The majority of the remaining 20% of oxygen generated commercially is utilized for water treatment, metal cutting, and welding, medicinal purposes, and as an oxidant in rocket fuel.
In oxyacetylene welding, oxygen is utilized to burn acetylene with oxygen to create an extremely high flame. Using a tiny oxy-acetylene flame, metal up to 60 cm (24 in) thick is heated in this technique before being swiftly sliced by a strong stream of oxygen.
Compounds
In nearly all known oxygen compounds, the oxidation state of oxygen is −2. There are certain molecules, such as peroxides, that have the oxidation state −1. Superoxides (−1/2), ozonides (−1/3), elemental hypofluorous acid (−0), dioxygenyl (+1/2), dioxygen difluoride (+1), and oxygen difluoride (+2) are very rare compounds containing oxygen in various oxidation states.
Oxides and Other Inorganic Compounds
The most well-recognized oxygen compound is water (H2O), which is an oxide of hydrogen. In addition to their covalent link with oxygen in a water molecule, hydrogen atoms are additionally attracted to a neighboring oxygen atom in a different molecule by around 23.3 kJ/mol.
Water molecules are held together by these hydrogen bonds around 15% more tightly than would be predicted in a basic liquid with only van der Waals forces.
Oxygen forms chemical bonds with practically all other elements to produce matching oxides because of its electronegativity.
Most metals, including titanium and aluminum, oxidize on their surfaces when exposed to air, producing a thin oxide coating that passivates the metal and inhibits more corrosion.
There is slightly less metal in many of the transition metal oxides than would be predicted from the chemical formula, making them non-stoichiometric combinations. For instance, the formula for the mineral FeO (wüstite) is 
Trace amounts of oxygen are found in the atmosphere as carbon dioxide (CO2). The oxides of silicon (silica SiO2, found in granite and quartz), and aluminum (aluminum oxide Al2O3, found in bauxite and corundum).
Iron (iron(III) oxide Fe2O3, found in hematite and rust), and calcium carbonate (found in limestone) make up a significant portion of the Earth’s crustal rock.
Oxygen compounds make up the remainder of the Earth’s crust as well, especially different complex silicates found in silicate rocks. Silicates of iron and magnesium make up the majority of the Earth’s mantle, which has a mass far greater than that of the crust.
Na4SiO4, Na2SiO3, and Na2Si2O5 are examples of water-soluble silicates that are utilized as adhesives and detergents.
Additionally, oxygen functions as a ligand for transition metals, combining with them to create transition metal dioxygen complexes with metal–O2.
Hemoglobin and myoglobin are heme proteins that belong to this family of substances. PtF6 undergoes an uncommon and exotic reaction where oxygen is oxidized to produceO2+PtF6−, dioxygenyl hexafluoroplatinate.
Organic Compounds
When “R” refers to an organic group, some of the most significant families of organic compounds containing oxygen are: alcohols (R-OH); ethers (R-O-R); ketones (R-CO-R); aldehydes (R-CO-H); carboxylic acids (R-COOH); esters (R-COO-R); acid anhydrides (R-CO-O-CO-R); and amides (R-CO-NR2).
Acetone, methanol, ethanol, isopropanol, furan, THF, diethyl ether, dioxane, ethyl acetate, DMF, DMSO, acetic acid, and formic acid are only a few of the significant organic solvents that include oxygen.
(CH3)2CO) acetone and phenol(C6H5OH) are utilized as starting materials for the synthesis of several other compounds. Acetic anhydride, glutaraldehyde, citric acid, glycerol, formaldehyde, and acetamide are other significant organic molecules that include oxygen.
Epoxides are ethers in which a ring of three atoms includes the oxygen atom. Similar to this, the element is present in nearly all biomolecules that are vital to life or produced by it.
Autoxidation is the spontaneous reaction of oxygen with numerous organic molecules at or below room temperature.
The majority of oxygen-containing chemical molecules are not directly created by O2. Peracetic acid and ethylene oxide are two examples of major organic chemicals used in trade and industry that are produced via direct oxidation of a precursor.
Safety and Precautions
Compressed oxygen gas is classified as an oxidant by the NFPA 704 standard, however, it is neither flammable, reactive, nor health-hazardous. The health hazard rating of refrigerated liquid oxygen (LOX) is 3 (due to the elevated danger of hyperoxia from condensed vapors and common cryogenic liquid hazards like frostbite); all other ratings are the same as those for compressed gas form.
Toxicity
At high partial pressures, oxygen gas (O2) can be harmful and cause convulsions as well as other health issues.
At partial pressures of more than 50 kilopascals (kPa), which is equivalent to around 50% oxygen composition at standard pressure or 2.5 times the typical sea-level O2 partial pressure of about 21 kPa, oxygen poisoning often starts to manifest.
As gas delivered through oxygen masks in medical applications usually consists of about 30–50% O2 by volume (approximately 30kPa at normal pressure), this is not a concern, with the exception of patients on mechanical ventilators.
Premature infants were once kept in incubators with O2-rich air, but this practice was abandoned since too much oxygen caused blindness in certain infants.
Because of the low overall pressures utilized, breathing pure oxygen in space applications—such as certain contemporary space suits or early spacecraft like Apollo—causes no harm.
The oxygen partial pressure in the breathing gas of astronauts wearing spacesuits is typically around 30 kPa, or 1.4 times normal. As a result, the oxygen partial pressure in the astronauts’ arterial blood is just slightly higher than the O2 partial pressure at sea level.
Surface-supplied diving and deep scuba diving can potentially result in oxygen toxicity to the central nervous system and lungs.
Persistent inhalation of an air mixture with more than 60kPa of O2 partial pressure can eventually cause irreversible lung fibrosis.
More above 160 kPa, or 1.6 atm, of O2 partial pressure, can cause convulsions, which are usually deadly for divers.
Breathing an air mixture containing 21% oxygen at a depth of 66 m (217 ft) or more can result in acute oxygen poisoning, which is the most dreaded impact for divers and can also happen when breathing 100% oxygen at a depth of only 6 m (20 ft).
Combustion and Other Hazards
Sources of oxygen that are highly concentrated encourage quick burning. Concentrated oxidants and fuels provide fire and explosion risks when they are placed close together; combustion requires an ignition event, such as heat or a spark.
The oxidant, not the fuel, is oxygen. Combustion can continue quickly and vigorously in the presence of concentrated O2.
Since steel pipes and storage containers used to transport and store both gaseous and liquid oxygen will burn like fuel, extra care must be taken throughout the design and production of O2 systems to reduce the possibility of ignition sources.
Instead of the 1⁄3 standard pressure that would be used on a flight, the capsule was pressurized with pure O2 but at slightly more than atmospheric pressure, which is why the fire that killed the Apollo 1 crew in a launch pad test spread so quickly.
If organic materials, such as wood, petrochemicals, and asphalt, are exposed to liquid oxygen spills, they may spontaneously explode upon subsequent mechanical impact.
FAQ
What is oxygen O or O2?
O2 is the formula for oxygen, and oxygen is represented by the symbol “O.” The symbol ‘O’ represents a free oxygen atom, while O2 is an oxygen molecule formed by the chemical bonding of two oxygen atoms. This is the distinction between the symbol and the formula.
What are the main uses of oxygen?
In order of significance, the following are the primary uses of oxygen: 1) the melting, refinement, and production of steel and other metals; 2) the controlled oxidation of chemicals; 3) rocket propulsion; 4) the support of biological and medical life; and 5) the mining, production, and manufacturing of stone and glass products.
Why is oxygen written as O2?
Since the periodic table describes atoms rather than molecules, the element is O. Since an oxygen molecule requires two O atoms to form, the molecule is designated as O2. Because the element exists in its pure form as a molecule made up of two atoms of the element, these kinds of elements are known as diatomic elements.
Which element is O2?
Diatomic (O2) and triatomic (O3, ozone) are the two allotropic forms of oxygen. The diatomic form’s characteristics imply that oxygen’s paramagnetism is caused by six electrons joining the atoms and two electrons remaining unpaired.
How is oxygen made?
The sea. Oxygen is produced by cyanobacteria, algae, and plants. They use photosynthesis to do this. They convert carbon dioxide and water into sugar and oxygen by harnessing the energy from sunshine.
What color is oxygen?
The gas has no flavor, smell, or color. Both the liquid and solid forms exhibit significant paramagnetic behavior and have a pale blue tint.
Where is oxygen found?
The earth and sky are largely composed of oxygen, which is our home planet. 46% of the Earth’s crust is made up of oxygen, mostly in the form of silicates, which are silicon and oxygen compounds. Molecular oxygen and ozone make up around 21% of the atmosphere’s total oxygen content.