Immunotherapy
What is an Immunotherapy?
Biological therapy, often known as immunotherapy, involves either stimulating or inhibiting the immune system to cure an illness.
Immunotherapies that diminish or suppress the immune system are categorized as suppression immunotherapies, whereas those that aim to stimulate or arouse the immune system are called activation immunotherapies.
The initial study is being done on immunotherapy’s potential to treat different types of cancer. For certain malignancies, immunotherapies based on cells work well.
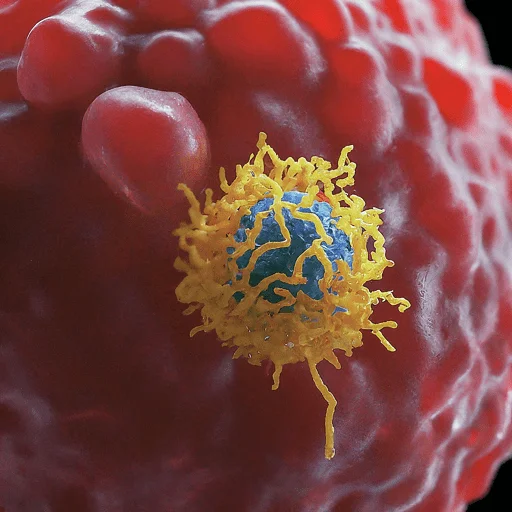
Targeting aberrant antigens produced on the surface of tumor cells, immune effector cells such as lymphocytes, macrophages, dendritic cells, natural killer cells, and cytotoxic T lymphocytes collaborate to protect the body against cancer.
The primary mechanism of COVID-19 vaccine-induced immunity is an immunomodulatory T-cell response. Medical use licenses have been granted for treatments including cellular membrane fractions from bacteria, imiquimod, interferons, and granulocyte colony-stimulating factor (G-CSF).
Other chemicals included in clinical and preclinical studies include glucans, synthetic oligodeoxynucleotides of cytosine phosphate-guanosine (CpG), IL-2, IL-7, IL-12, and other chemokines.
Immunomodulators
The immunotherapy’s active ingredients are known as immunomodulators. There is a wide range of natural, synthetic, and recombinant preparations.
| Class | Example agents |
| Interleukins | IL-2, IL-7, IL-12 |
| Tissue Kinases | Interferons, G-CSF |
| Chromokines | CCL3, CCL26, CXCL7 |
| Immunosuppressive medications | BCG vaccination, Covid vaccine, and thalidomide and its equivalents (pomalidomide, apremilast, and lenalidomide) |
| Other | Glutamate-cytosine phosphate, oligodeoxynucleotides, and glucans |
Activation Immunotherapies
Cancer
In the past, the goal of cancer treatment was to eradicate cancerous cells and tumors via radiation, surgery, or chemotherapy.
These therapies are still in use in many circumstances because they can be highly successful.
James P. The 2018 Nobel Prize in Physiology or Medicine was to Allison and Tasuku Honjo “for their discovery of cancer therapy by inhibition of negative immune regulation.”
The goal of cancer immunotherapy is to activate the immune system to eliminate tumors. Numerous tactics are being investigated and tested, or they are currently in use.
Cell-based immunotherapy is 20–30% more effective when paired with traditional treatment modalities.
Randomized controlled studies in various cancers have demonstrated a significant increase in survival and disease-free periods.
The BCG vaccine is one of the earliest examples of cancer immunotherapy. Originally developed to prevent tuberculosis, it was later discovered that bladder cancer could benefit from this vaccination as well.
Immune responses are elicited both locally and systemically by BCG treatment. Although much research has been done, the mechanisms by which BCG immunotherapy induces tumor immunity remain unclear.
Rituximab, an anti-CD20 antibody used to treat B cell lymphoma, was the first monoclonal antibody used in cancer therapy in 1997.
Since then, a number of monoclonal antibodies have been approved for the treatment of solid tumors and a variety of hematological malignancies.
G-CSF lymphocytes are drawn from the blood, grown in vitro against a tumor antigen, and then the cells are reinfused with the proper stimulatory cytokines.
Subsequently, the cells eliminate the tumor cells that exhibit the antigen. Through the use of an immune-enhancing cream called imiquimod, topical immunotherapy works to destroy warts, actinic keratoses, basal cell cancer.
Vaginal intraepithelial neoplasia, squamous cell cancer, cutaneous lymphoma, and superficial malignant melanoma by stimulating the recipient’s killer T cells to produce interferon.
Injection immunotherapy (also known as “intralesional” or “intratumorally”) treats warts (HPV-induced tumors) by injecting trichophyton antigen, the HPV vaccine, candida, or the mumps. Lung and other tumors have been tested for adoptive cell transfer; melanoma has shown the best results.
Dendritic cell-based pump-priming or vaccination
It is possible to excite dendritic cells (DC) to launch a cytotoxic response in response to an antigen. An antigen-presenting cell called a dendritic cell is taken from the immunotherapy patient.
These cells are subsequently transfected with a viral vector, pulsed with an antigen or tumor lysate, or both, so that they may express the antigen.
These activated cells give the antigen to the effector lymphocytes (B cells, cytotoxic CD8+ T cells, and CD4+ helper T cells) when they are transfusion into the patient.
As a result, tumor cells expressing the antigen are targeted for cytotoxicity, which Now primed as the adaptive reaction. One example of this strategy is the cancer vaccination Sipuleucel-T, which was the first cell-based immunotherapy to receive FDA approval.
This immunotherapy was created by the Immune Response Corporation, which licensed the technology to Dendron after it received FDA approval.
Currently used methods for DC-based vaccination involve injecting antigen-loaded DCs produced in vitro from monocytes or CD34+ cells back into patients after activating them with various TLR ligands and cytokine combinations.
The in vivo targeting techniques include the administration of particular cytokines (e.g., Flt3L, GM-CSF) and the targeting of DCs with agonistic antibodies (e.g., anti-C-type lectin receptor) or antibodies to C-type lectin receptors.CD40) that are coupled to the target antigen.
Subsequent strategies could focus on DC subsets according to the C-type lectin receptors or chemokine receptors that are uniquely expressed in them.
Making genetically modified DCs from induced pluripotent stem cells and using neoantigen-loaded DCs to improve clinical outcomes is another possible strategy.
T-cell Adoptive Transfer
Autologous, retrieved T cells are cultivated via adoptive cell transfer in vitro and then transfused.
As an alternative, T cells can be harvested and then infected with a retrovirus that carries a copy of the T cell receptor (TCR) gene, which is specialized to recognize cancer antigens. This process produces genetically altered T cells.
The receptor is incorporated into the T cells’ DNA by the virus. The cells are either activated or enlarged non-specifically. Following their reinfusion, the cells mount an immunological defense against the tumor cells.
The method has been applied to advanced skin cancer and resistant stage IV metastatic melanomas. Kymriah, the first CAR-T medication FDA-authorized, used this strategy.
Novartis acquired the manufacturing facility, the distribution network, and the production staff that created Sipuleucel-T, which was created by Dendreon and the Immune Response Corporation, in order to get the clinical and commercial supply of this CAR-T.
Before re-infusion, lympho-depletion of the recipient is necessary to remove regulatory T cells and unmodified, endogenous lymphocytes that compete with the transplanted cells for homeostatic cytokines, regardless of whether T cells are genetically engineered or not.
Myeloablative chemotherapy can be used to deplete lymph nodes; for maximum impact, total body radiation can be added.
Many recipients of transferred cells experienced in vivo cell multiplication and peripheral blood persistence; after 6–12 months following infusion, these cells occasionally accounted for 75% of all CD8+ T cells.
By 2012, Numerous locations were conducting metastatic melanoma clinical studies. Adoptive T-cell transfer produced clinical responses in patients whose metastatic melanoma was resistant to several immunotherapies.
Checkpoint Inhibitors
Patients can presently choose between two types of checkpoint inhibitors: anti-PD-1/PD-L1 and anti-CTLA-4 antibodies.
Anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and anti-programmed cell death protein 1 (PD-1) antibodies have already been approved for use in humans, and this has already led to notable changes in the course of some malignancies’ diseases.
Subsequent preclinical research revealed these molecules’ critical involvement in maintaining peripheral immunological tolerance, despite their initial discovery as molecules involved in T-cell activation or death.
Certain individuals with a range of cancer types, such as melanoma, breast, bladder, cervical, colon, head and neck, or Hodgkin lymphoma, are authorized to receive immune checkpoint inhibitor treatment.
These treatments have transformed cancer immunotherapy because they have improved overall survival for a growing number of patients, and they have shown, for the first time in many years of research, an improvement in immunogenicity in metastatic melanoma, one of the most immunogenic human cancers.
Immune Enhancement Therapy
Autologous immune enhancement therapy entails the in vitro multiplication and reintroduction of a patient’s own natural killer cells taken from peripheral blood, cytotoxic T lymphocytes, epithelial cells, and other relevant immune cells. The treatment has been evaluated for HHV6 infection, chronic fatigue syndrome, and hepatitis C.
Suppression Immunotherapies
Immune suppression lowers a normal immune response to avoid transplanted organ or cell rejection, or it dampens an aberrant immune response in autoimmune disorders.
Immunosuppressive Drugs
Immunosuppressive medications aid in the treatment of autoimmune diseases and organ transplants. Multiplication of lymphocytes is necessary for immune responses.
Immunosuppressive medicines are cytostatic. While immunophilin inhibitors more directly target T lymphocyte activation, glucocorticoids are a slightly more selective inhibitor of lymphocyte activation.
The immune response’s phases are targeted by immunosuppressive antibodies. Other medications regulate immune responses and have the potential to stimulate immunological control.
In a preclinical trial, it was found that the immune system’s control by tiny immunosuppressive molecules—like vitamin D, dexamethasone, and curcumin—administered subcutaneously and at low doses may be useful in avoiding or treating chronic inflammation.
Immune Tolerance
Naturally, the body doesn’t fight its own tissues using the immune system. CD4+ T-cells are typically identified by models as the focal point of the autoimmune response.
B-cells and other immune effector cells are then released onto the target tissue due to loss of T-cell tolerance. Targeting the particular T-cell clones coordinating the autoimmune onslaught would be the ultimate tolerogenic therapy.
In order to prevent the body from unintentionally attacking its own cells or organs in autoimmune diseases or from accepting alien tissue in organ transplants, immunological tolerance therapies aim to reset the immune system.
The introduction of regulatory immune cells into transplant recipients is a more recent[when?] therapeutic strategy. It is possible for the transfer of regulatory immune cells to impede effector action.
Building immunological tolerance lessens or completely removes the need for immunosuppression for the rest of one’s life and all of its negative effects. Type 1 diabetes, rheumatoid arthritis, transplants, and other autoimmune diseases have all been tested with it.
Allergies
Allergies can also be treated with immunotherapy. Immunotherapy can lower the severity of allergic reactions by reducing sensitivity to allergens, while allergy medications (such as corticosteroids or antihistamines) address the symptoms of allergies.
Immunotherapy might provide long-term advantages. Immunotherapy gives allergy sufferers an opportunity to lessen or completely eradicate their symptoms, however, it is ineffective in certain cases. Those with severe allergies or those who are unable to avoid particular allergens should consider the therapy.
Oral immunotherapy is a promising treatment option for food allergies (OIT). Through a gradual introduction of increasingly higher allergen doses, OIT can help most people tolerate food dosages high enough to avoid adverse reactions in the event of an inadvertent exposure.
As the person grows desensitized, dosages are increased over time. Babies have been tried with this method to prevent allergies to peanuts.
| Modality | Details | ||
| Non-antigen specific | Monoclonal Antibodies | Depleting: Anti-CD52 Anti-CD4 Anti-LFA-2 | Non-depleting: Anti-CD4 Anti-CD3 Anti-LFA-1 CTLA4-Ig Anti-CD25 |
| Haematopoietic stem cell transplantation | Non-myeloablative | Myeloablative | |
| Mesenchymal stem cell transplantation | |||
| Regulatory T cell therapy | Non-antigen specific | Antigen-specific | |
| IL-2 at low dose to increase regulatory T cells | |||
| Microbiome manipulation | |||
| particular antigen | peptide treatment | Subcutaneous, transmucosal, and intradermal (oral, breathed)Liposomes, nanoparticles, and tolerogenic dendritic cells | |
| Altered peptide ligands |
Helminthic Therapies
Tests have been conducted on hookworm (Necator americanus) and whipworm (Trichuris suis) ova for allergies and immunological disorders.
Treatments for relapsing remitting multiple sclerosis, Crohn’s disease, allergies, and asthma have all been studied, including helminthic therapy. It is uncertain how the helminths affect the immune response through a particular mechanism.
Theories include regulation of the Th1 / Th2 response and re-polarization of how dendritic cells work.
The helminths stimulate the production of regulatory Th2 cytokines like IL-10, IL-4, IL-5, and IL-13 while downregulating pro-inflammatory Th1 cytokines including interleukin-12 (IL-12), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α).
Some genes linked to interleukin expression and immunological illnesses, including Crohn’s, ulcerative colitis, and celiac disease, have been modified by coevolution with helminths.
The interaction between helminths and their human hosts can be categorized as either mutualistic or symbiotic.
FAQ
What is immunotherapy used to treat?
One kind of cancer treatment that boosts your immune system’s ability to combat cancer is immunotherapy. Your body uses the immune system as a weapon to fight against infections and other illnesses. It is made up of white blood cells, tissues of the lymphatic system, and organs. Biological therapy includes immunotherapy as a subtype.
At what stage of cancer is immunotherapy used?
When pembrolizumab and other ICIs were first introduced, they were exclusively prescribed to patients with extremely advanced malignancies that had stopped responding to conventional therapies. Nowadays, a number of them are utilized as first treatments for advanced tumors, including kidney and lung cancers, which have traditionally been difficult to treat.
Is immunotherapy a chemotherapy?
Two different pharmacological approaches are utilized to treat cancer: immunotherapy and chemotherapy. Increasing your immune system’s capacity to fight cancer cells is the aim of immunotherapy. Chemotherapy prevents cancer cells from proliferating on their own.
Is immunotherapy better than chemo?
Immunotherapy instructs healthy cells on how to identify cancerous cells and halt their spread. In other words, immunotherapy stimulates the immune system to fight cancer on its own, while chemotherapy directly destroys malignant cells and tumors.
Is immunotherapy Painful?
Skin reactions at the injection site, such as discomfort, swelling, and soreness, are the most frequent side effects for patients receiving immunotherapy medications intravenously. Although rare, some immunotherapy medications might result in serious or even fatal adverse responses.
How successful is immunotherapy?
Not everyone finds it effective. Certain malignancies respond better to immunotherapy medications than others, and while they can work like a miracle for some patients, not all of them will benefit from them. The average response rate is between 15% and 20%.
Who is eligible for immunotherapy?
If genomic testing reveals biomarkers that are positive for PD-L1 expression, high microsatellite instability, or high tumor mutational burden, you might be a candidate for immunotherapy. Your cancer has progressed.





3 Comments