Churg-Strauss Syndrome
What is Churg-Strauss syndrome?
Churg-Strauss syndrome, now known as eosinophilic granulomatosis with polyangiitis (EGPA), is characterized by inflammation of blood vessels, potentially leading to restricted blood flow and permanent damage to organs and tissues.
The predominant indicator of Churg-Strauss syndrome is adult-onset asthma, accompanied by various complications such as nasal allergies, sinus issues, rash, gastrointestinal bleeding, and pain and numbness in the extremities.
This rare disorder lacks a definitive cure, but symptoms can often be managed through the use of steroids and potent immunosuppressant medications.
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, represents a distinct subtype within the spectrum of diseases characterized by necrotizing vasculitis affecting small and medium-sized systemic blood vessels. Other subcategories include granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and polyarteritis nodosa. What sets EGPA apart is its unique combination of asthma, rhinosinusitis, and peripheral eosinophilia.
History:
In 1951, Jacob Churg and Lotte Strauss initially described this condition based on autopsy findings in 13 patients, all exhibiting a consistent pattern of severe asthma, fever, blood eosinophilia, and granulomatous necrotizing vasculitis. They termed it allergic granulomatosis and angiitis, with diagnostic criteria requiring the presence of eosinophilic infiltration, necrotizing vasculitis, and extravascular granuloma formation. Recognizing the variability of these features, Lanham et al. proposed a more clinically relevant definition, including bronchial asthma, significant blood eosinophilia, and vasculitis affecting at least two extrapulmonary organs.
In 1990, the American College of Rheumatology implemented revised categorization criteria that improved specificity and sensitivity by requiring the detection of four out of six characteristics for diagnosis. “Eosinophil-rich and granulomatous inflammation involving the respiratory tract, necrotizing vasculitis in small to medium-sized vessels, associated with asthma & eosinophilia” is the updated definition of EGPA that was agreed with at the Chapel Hill consensus meeting in 1994. This criterion eliminated the requirement for a biopsy, which facilitated the identification of early patients with only eosinophilia and asthma.
Churg-Strauss syndrome manifests through three key characteristics:
- Hypereosinophilia: This occurs when eosinophils, a specific type of white blood cells, aggregate within blood vessels and tissues.
- Vasculitis: Inflammation of blood vessels takes place, leading to restricted blood flow.
- Granulomatosis: Resulting from vasculitis, this process induces the formation of inflammatory nodular lesions known as granulomas.
The compromised blood flow poses a risk of organ damage, with the lungs being particularly vulnerable. If left untreated, this condition can have profound and detrimental effects on the body.
Alternative Names:
- Churg-Strauss vasculitis
- Allergic granulomatosis
- Allergic granulomatosis and angiitis
- Allergic angiitis and granulomatosis
Causes of Churg-Strauss syndrome
Recent endeavors to classify clinical phenotypes of Churg-Strauss syndrome (CSS) have focused on delineating vasculitic and eosinophilic subtypes, challenging the conventional nomenclature.
Patients testing positive for ANCA (reported in approximately 40% of all CSS cases) often exhibit a vasculitic phenotype, featuring myalgia, migrating polyarthralgia, weight loss, mononeuritis multiplex, and renal involvement, either as crescentic or necrotizing glomerulonephritis.
Conversely, those without ANCA positivity typically display an eosinophilic phenotype with a heightened incidence of myocarditis.
However, ANCA presence alone doesn’t strictly correlate with vasculitis. In a study by Cottin et al., around 47% of patients had vasculitis without ANCA seropositivity, and 29% were positive for myeloperoxidase ANCA without vasculitis.
Approximately 41% of patients in the same series did not exhibit vasculitis but rather an eosinophilic tissue infiltrate and involvement, highlighting the need to recognize subgroups with vasculitis features.
Vasculitic Phenotype:
Definitive vasculitis features include:
- Biopsy-proven necrotizing vasculitis of any organ
- Biopsy-proven necrotizing glomerulonephritis or crescentic glomerulonephritis
- Palpable purpura
- Alveolar haemorrhage
- Coronary arteritis causing myocardial infarction
Surrogates of vasculitis:
- Hematuria with red casts > 10%, dysmorphic RBC, and/or 2+ protein
- Leukocytoclastic capillaritis
- Mononeuritis
- Presence of ANCA.
The remainder falls into the eosinophilic asthma phenotype. Early recognition of this subgroup, especially in cases of myocarditis without evidence of vasculitis, is crucial. Relying solely on vasculitis for CSS diagnosis may risk overlooking those with eosinophilic asthma who could benefit from early targeted therapy.
Pathogenesis:
At the cellular level, an aberrant T-helper cell pathway appears to be the primary trigger. The Th2 cell lineage plays a pivotal role, with theories suggesting various factors such as allergies, infections, and medications. While the allergy theory lacks robust evidence, a superantigen theory activating specific T cell subsets and airway colonization by Aspergillus or Actinomyces has been proposed. Leukotriene inhibitors, particularly montelukast and zafirlukast, have shown a strong association with CSS onset, as reported in FDA data.
Specific clonally expanded T cell subpopulations and the increased frequency of related HLA alleles further support the pathogenic role of T cells. Elevated serum IL-10 levels, mediating Th-1 response inhibition, contribute to Th2 cell differentiation, particularly in the ANCA-negative phenotype of CSS.
Epidemiology of Churg-Strauss Syndrome
CSS prevalence is estimated at 10.7 to 14 per million adults worldwide. Onset typically occurs between 38 and 54 years, with extremes ranging from 4 to 74. No gender difference in incidence is observed.
Unlike small-vessel vasculitis, which is more common in childhood, CSS is rare in children. Regardless of age, both adult and childhood CSS present with elevated IgE and eosinophil levels. About 40% of adults have positive ANCA, while 25% of children are seropositive. Childhood CSS, clinically distinct with a higher incidence of cardiomyopathy, pneumonic infiltrates, and rarer mononeuritis multiplex, exhibits increased mortality due to a higher prevalence of cardiac disease.
Pathophysiology of Churg-Strauss Syndrome
The underlying mechanisms and clinical manifestations of Churg-Strauss syndrome (CSS) exhibit a dual nature, involving either eosinophil-mediated damage or endothelial injury induced by antineutrophil cytoplasmic antibodies (ANCA).
Eosinophils:
Initiated by a TH2-mediated immune response, eosinophil margination is provoked. Elevated synthesis, enhanced extravasation, and prolonged survival of eosinophils in target tissues characterize active disease. TH-2 lymphocyte-produced IL-3 and IL-5 play pivotal roles in regulating eosinophil maturation, release, and survival. Disease activity correlates consistently with serum IL-5 levels, which decrease with immunosuppressive therapy initiation.
Epithelial and endothelial cells, activated by Th-2 cytokines, release eosinophil-specific chemokines, including eotaxin 3 (CCL26), CCL17, and CCL22. These chemokines act on CCR4 receptors, facilitating the recruitment of eosinophils and Th2 cells to end organs and amplifying the immune response. Serum CCL26 levels serve as a reliable marker for disease activity in CSS.
Eosinophils release cationic proteins like eosinophil cationic protein (ECP), eosinophil peroxidases, eosinophil-derived neurotoxins, and eosinophil granule major basic protein, directly contributing to tissue damage. They also secrete cytokines such as IL-1, IL-3, IL-5, TGF-beta, and vascular endothelial growth factor. IL-5 actively participates in eosinophil maturation, differentiation, and survival.
Histological findings in CSS include eosinophilic infiltrates in small to medium-sized blood vessels and extravascular tissue spaces. Acute pulmonary exacerbations exhibit bronchoalveolar lavage fluid rich in eosinophils, akin to acute or chronic eosinophilic pneumonia. Extravascular eosinophilic granulomas are observed, particularly in the gastrointestinal tract.
IL-5 is not the sole mediator of eosinophilic tissue infiltration, as evidenced by tissue Major Basic Protein (MBP) persistence despite mepolizumab therapy downregulating IL-5 titers. Th-2 cytokines IL-4 and IL-13 may also play essential roles in tissue infiltration and eosinophil degranulation.
Peripheral blood eosinophils in CSS express activation markers CD69 and CD25, accompanied by increased serum IL-5 and ECP.
ANCA:
Approximately 40% of CSS patients exhibit elevated perinuclear ANCA (p-ANCA) levels, with antibodies in a perinuclear pattern. Rarely, a cytoplasmic pattern with specificity for neutrophil proteinase 3 (c-ANCA) is observed.
ANCA presence correlates with an increased incidence of glomerulonephritis, mononeuritis, and biopsy-proven vasculitis. Alveolar haemorrhage is more prevalent in this group.
Infusion of myeloperoxidase (MPO)-ANCA in mice results in severe necrotizing and crescentic glomerulonephritis. The two-subset hypothesis in CSS clinical phenotyping is further supported by the increased HLA-DRB4 frequency in ANCA-positive CSS patients. Recent evidence suggests the involvement of Th17 lymphocytes in the vasculitis response, emphasizing the balance between Th17 and Treg cells.
Endothelial injury in ANCA-associated vasculitis is neutrophil-mediated, involving the generation of reactive oxygen species and proteolytic enzymes from cytoplasmic granules.
Symptoms of Churg-Strauss Syndrome
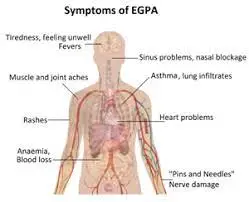
Churg-Strauss syndrome primarily impacts the lungs but can extend to various organ systems. The manifestation of symptoms is contingent upon the affected organs, with a majority of patients exhibiting asthma or asthma-like symptoms. A distinctive hallmark is the elevated presence of eosinophils, a type of white blood cell, termed hypereosinophilia.
Typically released during allergic reactions or parasitic infections, eosinophils accumulate excessively in the tissues of individuals with Churg-Strauss syndrome. The symptoms align with the organ systems harbouring concentrated eosinophils. For instance, pulmonary symptoms arise when eosinophils accumulate in the lungs, while gastrointestinal symptoms manifest when the cells accumulate in the intestines.
Symptoms are categorized into three phases, but their sequence and occurrence can vary. Recognizing these phases is crucial for early diagnosis and intervention to prevent the progression of later stages.
The Three Phases:
- Prodromal Phase: Initiated by the development of asthma or asthma-like symptoms, this phase may extend over months, years, or decades.
- Eosinophilic Phase: Marked by the release of excessive eosinophils, this phase sees their accumulation in various organs, such as the lungs, intestines, or skin, eliciting corresponding symptoms.
- Vasculitic Phase: Widespread inflammation in blood vessels, known as vasculitis, characterizes this phase. Chronic inflammation poses the risk of complications like aneurysms, accompanied by symptoms of pain, bleeding, joint pain, weight loss, rashes, numbness, or tingling.
Varied Symptoms:
Symptoms during the prodromal phase typically involve respiratory issues, including an itchy or runny nose, sinus pressure, nasal polyps, and coughing or wheezing.
In the eosinophilic phase, symptoms become more generalized, encompassing fatigue, night sweats, abdominal pain, and fever.
The vasculitic phase introduces inflammatory symptoms, such as joint pain, weight loss, rashes, numbness, tingling, and muscle pain. Severe manifestations may occur if organs like the heart and kidneys are affected, with approximately 78% of patients experiencing neurological symptoms, including polyneuropathy.
Risk factors for Churg-Strauss syndrome
Churg-Strauss syndrome may manifest in individuals of any age or gender, with an average onset age ranging from 38 to 54 years. Notably, the condition can affect a diverse age range, spanning from 7 to 74 years.
While there are no distinct risk factors for the initial development of Churg-Strauss syndrome, some factors may increase the likelihood of a relapse, including:
- Recurring antineutrophilic cytoplasmic antibody (ANCA) positivity, indicating autoimmune vasculitis.
- An elevation in ANCA levels.
- Gastrointestinal tract (GI) problems
- A sudden increase in eosinophil count.
Recurrence:
Churg-Strauss syndrome carries a 20–30% chance of recurrence.
Related Conditions
Several conditions share similarities with Churg-Strauss syndrome, making a careful differential diagnosis crucial. These related conditions include:
- Wegener’s Granulomatosis: similar in nature but predominantly affecting the sinuses.
- Polyarteritis nodosa: Causes weakening and degeneration of blood vessels.
- Guillain-Barré Syndrome: Often leading to paralysis.
Drawing distinctions in signs and symptoms across these related conditions aids doctors in producing an accurate and specific differential diagnosis.
Diagnosis and Physical Assessment
Churg-Strauss syndrome diagnosis hinges on a comprehensive assessment, incorporating symptom history, imaging (X-rays, CT scans), and blood tests gauging eosinophil levels. Occasionally, tissue biopsies examine organ-specific eosinophil concentrations.
The American College of Rheumatology outlines diagnostic criteria, aiding in differentiating Churg-Strauss from other vasculitis types. Criteria encompass:
- Asthma
- Eosinophilia
- Mono- or polyneuropathy
- Nonfixed pulmonary infiltrates
- Abnormal paranasal sinuses (e.g., nasal polyps)
- Extravascular eosinophilia
History and Physical Examination:
Churg-Strauss unfolds in distinct phases, with features of eosinophilic tissue infiltration and small- to medium-vessel vasculitis.
- Prodromal Phase:
- Characterized by malaise, fever, polyarthralgia, and weight loss.
- Adult-onset asthma, refractory to conventional treatment.
- Upper respiratory symptoms, chronic rhinosinusitis, and nasal polyps.
- Eosinophilic Phase:
- Eosinophilic infiltrates in end organs with peripheral eosinophilia.
- Pulmonary manifestations include nodular infiltrates and eosinophilic gastroenteritis.
- Vasculitic Phase:
- The onset of vasculitis, occurring 3 to 9 years post-asthma onset.
- Neurological symptoms become prominent.
Organ System Involvement:
- Respiratory and Pulmonary Manifestations:
- Adult-onset asthma with an eosinophilic phenotype.
- Chronic rhinitis, sinusitis, and nasal polyposis.
- Pulmonary manifestations include transient infiltrates and eosinophilia.
- Cardiac Disease:
- Present in 62% of cases, symptomatically in 26%.
- Myocarditis leads to fibrosis, restrictive cardiomyopathy, and cardiac failure.
- Echocardiography and cardiac MRI aid in detection.
- Gastrointestinal Involvement:
- Eosinophilic gastroenteritis and mesenteric vasculitis.
- Manifestations range from abdominal pain to intestinal obstruction.
- Renal Involvement:
- Seen in 25% of patients, often with necrotizing glomerulonephritis.
- Hypertension may indicate renal involvement.
- Neurologic Involvement:
- Mononeuritis multiplex, peripheral neuropathy.
- CNS vasculitis causes cerebral infarctions.
- Autonomic neuropathies are implicated in cardiac dysrhythmia.
- Other organs:
- Dermatological manifestations occur in half to two-thirds of patients.
- Extravascular granulomas are observed in the skin.
- Various rare involvements, like central retinal artery occlusion and vasculitis in breasts.
Evaluation:
- Diagnosis relies on clinical features, peripheral blood eosinophilia (>10%), and elevated serum IgE.
- ANCA positivity in approximately 40% of cases
- Imaging (CT chest, sinuses) aids in revealing characteristic patterns.
- Using cardiac MRI to identify cardiac involvement early
- Sural nerve biopsy for peripheral neuropathy confirmation
- Skin biopsy, revealing small vessel vasculitis, aids in diagnosis, especially when combined with other manifestations.
Treatment of Churg-Strauss Syndrome
Early identification and the judicious use of corticosteroids and immunosuppressants have markedly altered the course of Churg-Strauss syndrome (CSS), enhancing prognosis and overall survival.
- Corticosteroids: These mitigate eosinophil burden in blood and tissues, impeding prolonged eosinophil survival in extravascular spaces. Initial therapy for non-severe cases involves 1 mg/kg of oral prednisone. Severe cases may benefit from pulse-dose methylprednisolone, sometimes coupled with cyclophosphamide.
- French Vasculitis Study Group Trials: Standardised treatment, based on a 5-factor score, revealed that corticosteroids alone induced remission in 93% of patients without poor prognostic factors. Relapses prompted additional agents like cyclophosphamide or azathioprine for maintenance.
- Refractory Disease Strategies:
- Plasmapheresis: effective in rapidly progressive glomerulonephritis or alveolar haemorrhage.
- Intravenous immunoglobulin (IVIG): considered for refractory neuropathy or cardiomyopathy.
- Biological Agents: Rituximab and tumour necrosis factor inhibitors (TNF) are alternatives, supported by success in other ANCA-associated vasculitides.
- Interferon-alfa shows promise in inducing remission, particularly in patients refractory to cyclophosphamide.
- Novel Therapies: Mepolizumab and omalizumab have shown success in refractory cases, previously used for severe persistent asthma.
- Treatment Plan Based on Ribi et al.:
- Induction of Remission:
- Without poor prognosis: oral prednisone tapering or intravenous methylprednisolone pulse.
- Relapse: oral azathioprine or cyclophosphamide pulses.
- With Poor Prognosis: Methylprednisolone Pulses with Additional Cyclophosphamide
- Maintenance of Remission:
- Options include methotrexate, cyclosporin A, or azathioprine.
- Induction of Remission:
- Refractory Disease Options:
- Plasma exchange, IVIG, interferon-alfa, TNF inhibitors (infliximab, etanercept, adalimumab), and Rituximab are considered.
Despite well-established choices, challenges persist in determining the ideal treatment duration and the sequence for discontinuing medications, emphasizing the ongoing complexity of managing Churg-Strauss syndrome.
Prognosis of Churg-Strauss Syndrome
Upon timely detection and intervention, Churg-Strauss syndrome (CSS) exhibits a favorable prognosis, boasting a 5-year survival rate of 90%.
Relapse Dynamics: Relapses, observed in 20–30% of cases, typically present with minor symptoms like fever, joint pain, and constitutional manifestations.
Risk Factors for Relapse:
- Sudden elevation in eosinophil count.
- Persistent ANCA positivity.
- Gastrointestinal tract (GI) involvement
- Increase in ANCA titers.
Association with Peripheral Eosinophilia: The degree of eosinophilia correlates with the extent of vasculitic disease, with a surge in eosinophil counts heralding vasculitis relapse.
Factors Influencing Mortality (Guillevin et al.):
- Proteinuria (>1 g per day)
- Renal insufficiency (Cr > 1.58 mg/dl)
- Cardiomyopathy
- GI tract involvement
- CNS involvement
Five-Factor Score (FFS):
- Defined by the presence of factors contributing to higher mortality.
- The absence of any factor signifies a good prognosis.
- The presence of two or more factors elevates the mortality risk.
Independent Risk Factor: Cardiomyopathy emerges as an independent risk factor in CSS (hazard ratio 3.39).
Clinical Assessment Tools:
- Birmingham Vasculitis Activity Score (BVAS): A detailed tool evaluating vasculitic organ involvement commonly employed in clinical trials
- Vasculitis Damage Index (VDI): assesses cumulative organ damage, reflecting both disease and treatment impacts. Correlates strongly with mortality and morbidity.
Factors Associated with a Poor Prognosis:
- Severe GI tract involvement.
- Cardiomyopathy.
- CNS vasculitis.
- Renal failure.
Cardiac Involvement: Emerges as a predominant cause of mortality, particularly in cases with a suboptimal response to therapy.
Navigating through these prognostic factors and utilizing comprehensive assessment tools is crucial for effective management and understanding the varied clinical trajectories in Churg-Strauss syndrome.
Complications of Churg-Strauss Syndrome
Churg-Strauss syndrome can impact diverse organs, encompassing the lungs, sinuses, skin, gastrointestinal system, kidneys, muscles, joints, and heart. This illness may be fatal if ignored.
Complications, contingent on affected organs, may involve:
- Peripheral Nerve Damage:
- Churg-Strauss syndrome may inflict damage on peripheral nerves in the hands and feet.
- Manifestations encompass numbness, a burning sensation, and loss of function.
- Heart Disease:
- Cardiovascular complications include inflammation of the heart membrane, muscular layer inflammation, heart attacks, and heart failure.
- Kidney Damage:
- Affection of the kidneys can result in glomerulonephritis.
- Impaired kidney filtering function leads to the accumulation of waste products in the bloodstream.
- Respiratory Complications:
- Lung involvement may cause respiratory distress and compromise pulmonary function.
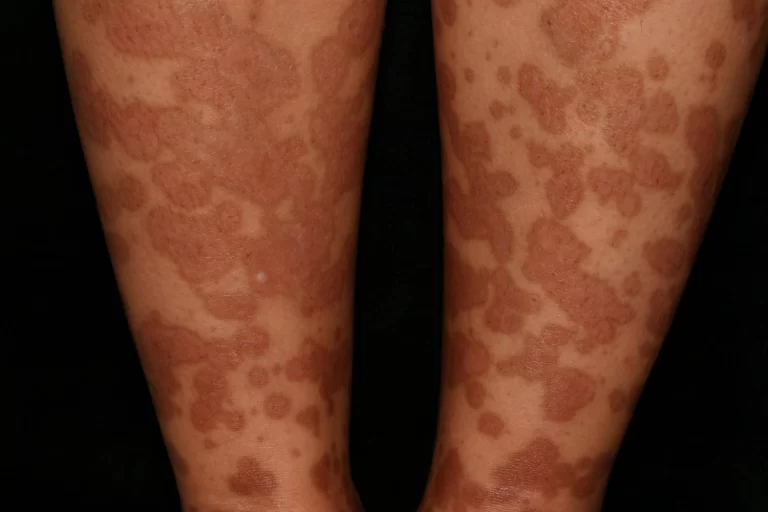
- Skin Disorders:
- Skin complications range from rashes to vasculitic lesions, affecting the overall dermatological health.
- Gastrointestinal Involvement:
- Gastrointestinal manifestations can vary from abdominal pain to more severe issues like bleeding or intestinal obstruction.
- Muscle and joint impairment:
- Muscular and joint complications may result in pain, swelling, and restricted mobility.
- Systemic Impact:
- The systemic nature of Churg-Strauss syndrome underscores the potential for multi-organ involvement, necessitating comprehensive medical attention.
Mortality Risk:
- Left untreated, Churg-Strauss syndrome poses a significant risk of mortality due to the progressive nature of complications.
Understanding and managing these complications is pivotal in enhancing the overall prognosis and quality of life for individuals grappling with Churg-Strauss syndrome.
Promoting Awareness and Patient Guidance for Churg-Strauss Syndrome
Formerly recognized as Churg-Strauss syndrome, this condition is a complex immune system disorder with systemic implications.
- Early Warning Signs:
- Vigilance is crucial when poorly controlled asthma persists for months, accompanied by sinus and nasal symptoms, especially alongside elevated blood eosinophil counts.
- Timely intervention matters.
- Initiate treatment promptly with steroids and other immunosuppressants for a favorable prognosis and extended life expectancy.
- Lingering Effects and Lifelong Management:
- Some effects, like bronchial asthma and certain muscle and nerve weaknesses, may persist throughout life.
- Regular, careful monitoring for adverse effects stemming from prolonged corticosteroid use is imperative, considering potential long-term treatment requirements.
- Proactive Healthcare Engagement:
- Adherence to diagnostic procedures and treatment plans is paramount. Regular follow-ups with healthcare providers post-diagnosis should not be overlooked, ensuring ongoing management and support.
By recognizing early indicators, promptly addressing symptoms, and actively participating in regular healthcare check-ins, individuals can empower themselves to navigate the complexities of Churg-Strauss syndrome while optimizing their overall health and well-being.
Adapting to Lifestyle Changes with Churg-Strauss Syndrome
In navigating long-term steroid use for EGPA, adopting certain lifestyle adjustments becomes integral to managing potential side effects.
- Prioritise bone health:
- Consult your doctor regarding vitamin D and calcium intake, assessing whether dietary adjustments or supplements are necessary.
- Embrace physical activity:
- Counteract steroid-induced weight gain through regular aerobic exercises like walking or swimming.
- Incorporate strength training and weight-bearing exercises to fortify bones.
- Quit smoking:
- Eliminate this detrimental habit, as it not only independently impacts health but can exacerbate steroid side effects.
- Opt for nutrient-rich foods:
- Counteract potential blood sugar elevation by choosing a diet rich in lean protein, fruits, whole grains, and vegetables.
- Regular medical monitoring:
- Engage in regular check-ups with your doctor to ensure vigilant monitoring during treatment and prevent relapses.
- Anticipate assessments such as X-rays, eye exams, blood pressure readings, blood sugar tests, and cholesterol checks.
Proactively incorporating these lifestyle modifications contributes to a more holistic approach to managing Churg-Strauss syndrome, fostering overall well-being amidst the challenges posed by extended steroid usage.
Enhancing Management Team Outcomes
In the current era of healthcare cost containment and the growing prominence of independent urgent care centers, it’s imperative to acknowledge exceptions to Occam’s razor principle.
- Recognizing Patterns for Comprehensive Care:
- The persistence of asthma exacerbations despite standard therapy warrants a comprehensive approach.
- Broadening suspicion and conducting initial blood work, including cell differentials, ESR, CRP, and IgE, aids in early detection.
- Correlating organ involvement like cardiomyopathy, renal disease, or GI symptoms with eosinophilic asthma contributes to a unified diagnosis of Churg-Strauss syndrome.
- Discontinuation Considerations:
- In cases of eosinophilic asthma with multisystem involvement, evaluating the role of leukotriene receptor antagonists in pathogenesis is essential.
- Prompt recognition and treatment significantly impact prognosis and life expectancy.
- Post-Diagnosis Care Focus:
- After diagnosis, awareness of potentially prolonged steroid dependence underscores the importance of regular glycemic status, bone density, fall prevention, and infection prevention screenings.
- An interprofessional team, including primary care providers, specialists, nurse practitioners, and pharmacists, collaborates to enhance patient outcomes.
- Pharmacists play a pivotal role in dosing, drug interactions, and patient education, while nurses administer treatment, monitor side effects, and provide crucial education to patients and their families.
In this collaborative effort, the interprofessional team ensures seamless coordination, improving the overall management of Churg-Strauss syndrome for better patient outcomes.
FAQ
What is the Churg-Strauss syndrome triad?
The rare condition known as Churg-Strauss’ syndrome, or allergic granulomatous angiitis, is typified by the co-occurrence of hypereosinophilia, pulmonary infiltrates, and asthma.
What is the cause of Churg-Strauss syndrome?
Serious organ damage and possibly fatal consequences could arise from improper treatment. While the precise aetiology of Churg-Strauss syndrome remains unclear, numerous investigators suggest that aberrant immune system operation is a significant contributing factor.
What is the life expectancy of Churg-Strauss syndrome?
In general, the 5-year survival rate in EGPA is roughly 25% in the absence of treatment. The 1-year survival rate is 90% and the 5-year survival rate is 62% after therapy.
What are the six criteria for the diagnosis of Churg-Strauss syndrome?
Asthma, peripheral eosinophilia greater than 10%, mono- or polyneuropathy, transient pulmonary infiltration, an anomaly of the paranasal sinuses, and histology demonstrating arteries with tissue eosinophilia are the six diagnostic criteria for CSS.
What is the new name for Churg-Strauss syndrome?
The condition known as eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss Syndrome) is brought on by inflammation (swelling) in specific cell types found in your tissues or blood.
What are the blood markers for Churg-Strauss syndrome?
Doctors use one or more of the following tests to diagnose Churg-Strauss syndrome: blood examinations. Your doctor could instruct them to count the eosinophils in your blood or look for indications of inflammation throughout your body. Elevated eosinophil counts may indicate the presence of asthma or Churg-Strauss syndrome.
Can you live a long life with Churg-Strauss?
Churg-Strauss syndrome used to be deadly in 70%–90% of cases within months. This indicates that 70–90% of individuals are still alive five years after diagnosis. Individuals above 65 have a worse outlook.
What does Churg-Strauss rash look like?
Palpable purpura, a macular or papular erythematous rash, haemorrhages (petechiae to ecchymoses), and sensitive cutaneous or subcutaneous lumps, frequently from granulomas, are among the skin symptoms of Churg-Strauss syndrome.
Can Churg-Strauss syndrome be cured?
Churg-Strauss syndrome, or eosinophilic granulomatosis with polyangiitis (EGPA), is incurable. Drugs can aid in maintaining your symptoms.
Reference
- Chakraborty, Rebanta K. “Churg-Strauss Syndrome.” StatPearls – NCBI Bookshelf, 23 Mar. 2023, www.ncbi.nlm.nih.gov/books/NBK537099.
- “Churg-Strauss Syndrome – Symptoms and Causes – Mayo Clinic.” Mayo Clinic, 24 Sept. 2021, www.mayoclinic.org/diseases-conditions/churg-strauss-syndrome/symptoms-causes/syc-20353760.
- Professional, Cleveland Clinic Medical. “EGPA (Formerly Churg-Strauss Syndrome).” Cleveland Clinic, my.clevelandclinic.org/health/diseases/7098–eosinophilic-granulomatosis-with-polyangiitis-egpa-formerly-churg-strauss-syndrome. Accessed 20 Nov. 2023.
- “Churg Strauss Syndrome – Vasculitis UK.” Vasculitis UK, 24 Aug. 2019, www.vasculitis.org.uk/about-vasculitis/churg-strauss-syndrome.
- Millar, Helen. What to Know About Churg-Strauss Syndrome. 31 Oct. 2022, www.medicalnewstoday.com/articles/churg-strauss-syndrome#outlook.
- “Osmosis – Churg-Strauss Syndrome: What Is It, Causes, Diagnosis, Treatment, and More.” Osmosis, www.osmosis.org/answers/Churg-Strauss-syndrome. Accessed 20 Nov. 2023.
- “Eosinophilic Granulomatosis With Polyangiitis (EGPA).” WebMD, 3 Dec. 2003, www.webmd.com/lung/egpa.
- Norman, Abby. “An Overview of Churg Strauss Syndrome.” Verywell Health, 26 May 2022, www.verywellhealth.com/churg-strauss-sydrome-4174962.



One Comment