Lambert-Eaton syndrome
What is Lambert-Eaton Syndrome?
Lambert-Eaton syndrome, also known as Lambert-Eaton myasthenic syndrome, the immune system attacks neuromuscular junctions, or the locations where your nerves and muscles connect. Normally, your nerve cells send information to your muscle cells. These impulses help contract the muscles. Having Lambert-Eaton syndrome makes it difficult for your muscles to move as naturally as they normally would because it impairs the nerves’ ability to connect with them.
In the rare presynaptic disorder of neuromuscular transmission known as Lambert-Eaton myasthenic syndrome (LEMS), the quantal release of acetylcholine (ACh) is impaired, leading to a distinctive set of clinical features such as autonomic alterations, post-tetanic potentiation, suppressed tendon reflexes, and proximal muscular weakness.
Although the two illnesses proceed in somewhat different ways, their early presentations might be comparable to those of myasthenia gravis (MG) and myasthenia minor (MM).
The neuromuscular junction (NMJ)’s ordinarily dependable neurotransmission is disturbed by LEMS. An autoantibody-mediated ablation of a portion of the P/Q-type Ca2+ channels involved in neurotransmitter release is suggested to be the cause of this disturbance.
Cancer is either present when the weakening starts or is discovered later in 40% of persons with LEMS. Although LEMS has also been linked to non-SCLC, lymphosarcoma, malignant thymoma, or carcinomas of the breast, stomach, colon, prostate, bladder, kidney, or gallbladder, this often refers to small cell lung cancer (SCLC).
Clinical manifestations frequently precede cancer identification. Cancer is frequently detected during the first two years after the initiation of LEMS, and almost always within four years.
Pathophysiology
Clinical data suggested an autoimmune etiology for LEMS. These observations included some of the following:
LEMS is frequently connected to well-known autoimmune diseases.
Effective therapies include prednisone, plasma exchange (PEX), and intravenous immunoglobulin (IVIg).
Organ-specific autoantibodies typically have increased serum levels in LEMS patients who do not have malignancy.
The autoimmune etiology of LEMS has acquired more concrete data to support it. On the presynaptic muscle membrane, active zone particles (AZPs), which stand in for voltage-gated calcium channels (VGCCs), are often packed in regular parallel arrays. Divalent antibodies against the VGCC cross-link the calcium channels, destroying the parallel arrays in LEMS patients and mice injected with LEMS immunoglobulin G (IgG). In the end, the AZPs cluster and become less numerous.
SCLC cells are made of neuroectoderm, share several antigens with tissue from the peripheral nervous system, and have high levels of VGCCs. LEMS IgG blocks calcium influx into these cells. Many LEMS patients have VGCC antibodies in their serum. These findings imply that VGCC antibodies suppress VGCCs in LEMS.
Cancer cells in LEMS patients with SCLC or other cancers are likely to possess antigens that resemble VGCCs and trigger the development of VGCC antibodies. Voltage-gated calcium channel (VGCC) antibodies are likely created as a component of a more general autoimmune condition in people with LEMS but no malignancy. An antibody response to domain IV of the 1A subunit of P/Q-type voltage-gated calcium channels vgcc is present in patients with LEMS who do not have malignancy.
Antibody response to domain IV of the 1A subunit of P/Q-type voltage-gated calcium channels VGCCs is more frequent in patients with LEMS without cancer than it is in patients with LEMS with cancer.
Calcium voltage-gated channels In patients with LEMS, VGCC antibody levels do not correspond to illness severity. However, when a patient’s condition becomes better after receiving cancer treatment or immunosuppression, their antibody levels do drop.
All LEMS patients with concomitant SCLC had a long history of smoking. Only 50 percent of people with autoimmune LEMS have smoked for a long time.
Epidemiology
Unknown is the actual incidence of LEMS. LEMS is thought to be present in 3% of SCLC patients. The real overall prevalence of LEMS may be much greater because only 50–70% of people with LEMS have cancer that can be identified and because LEMS frequently remains undetected in patients.
SCLC makes up the vast majority of tumors connected to LEMS. But there could be a variety of cancers at play. Non-SCLC, neuroendocrine carcinomas, lymphosarcoma, malignant thymoma, cancers of the breast, stomach, colon, prostate, bladder, kidney, and rectum, basal cell carcinoma, leukemia, lymphoproliferative diseases such as Castleman syndrome, and Hodgkin lymphoma are only a few of the diseases on the limited list.
Approximately 400 instances are thought to be present at any given moment in the United States. The number of LEMS patients who do not have SCLC or any other recognizable cancer is not considered in our estimation, though.
demographics connected to age and sex
LEMS is primarily a condition of middle-aged and older adults and typically manifests in later adulthood. The age of 60 is when symptoms typically start to develop. Although it is uncommon in kids, at least 7 kids under the age of 17 are said to have had LEMS.
According to past reports, LEMS affected men more frequently than women, roughly 2:1. However, according to recent findings, men and women experience it virtually equally.
What causes Lambert-Eaton syndrome?
Small-cell lung cancer is a particular form of cancer that is frequently linked to this illness. Your body may experience this condition as a result of its efforts to treat the underlying malignancy.
Lambert-Eaton syndrome develops following another autoimmune disease in some of the remaining cases. Sometimes the cause is not known.
LEMS is often associated with lung cancer (50–70%), specifically small-cell carcinoma, making LEMS a paraneoplastic syndrome. LEMS is seen in 1-3% of patients with small-cell lung cancer. LEMS is typically the initial sign of lung cancer in these situations, and it is otherwise asymptomatic.
LEMS may also be connected to endocrine conditions such as type 1 diabetes mellitus and hypothyroidism, a condition in which the thyroid gland is underactive. Similar genetic mutations that appear to predispose people to both LEMS without a tumor and myasthenia gravis without a tumor are prevalent in the two types of patients. Another possible cause of myasthenia gravis is a thymoma, a tumor of the thymus in the chest. HLA-DR3-B8 in particular appears to be prone to LEMS.
Symptoms of Lambert-Eaton syndrome
The patient will usually be an adult male over the age of fifty, presenting with either of the following:
Weakness: It frequently affects the muscles in the lower proximal limbs. Weakness gradually moves in the cranial direction and from proximal to distal muscle units. As a result, the upper limbs become gradually implicated, and finally, the oculobulbar region is impacted. In LEMS, muscle weakness is typically
correlated with severe impairment. The “Lambert’s Sign,” in which the grip gets stronger after several assessments of strength, can be elicited by the therapist. Heat and exhaustion exacerbate weakening.
Depressed or absent deep tendon reflexes are a typical symptom of hyporeflexia. After exercise, the patient can exhibit normoreflexia, though.
Common symptoms of autonomic dysfunction include constipation, orthostatic hypotension, dry mouth, blurred vision, and poor sweating.
Bulbar involvement is an uncommon condition that can manifest as dysphagia, diplopia, or ptosis. Ptosis and ophthalmoplegia are far less severe in LEMS than they are in MG.
Respiratory Failure: This very rare condition develops quite late in the course of the illness.
Trouble walking
Tingling sensation in the hands or feet
Eyelid drooping
Fatigue
Dry mouth
Trouble speaking and swallowing
Trouble breathing
Bladder and bowel changes
Erectile dysfunction
Signs:
The muscles in the proximal arms and legs, or those that are closest to the trunk, are frequently affected by LEMS weakness. Unlike myasthenia gravis, the weak point
More effect on the legs than the arms. This makes it difficult to get up from a sitting position and ascend stairs. In many cases, weakness is temporarily reduced after
physical effort or workout. The symptoms may get worse at high temperatures. Sometimes people experience weakness in their bulbar muscles, which include their neck and mouth muscles.
The ocular muscles rarely become weak. Some may experience trouble swallowing, double vision, or drooping of the eyelids, but usually only in combination with limb weakness;
This further sets LEMS apart from myasthenia gravis, which has far more frequent eye symptoms. The disease’s advanced stages are marked by the weakness of the respiratory muscles may occur. Some people may also have ataxia, which causes issues with coordination.
Autonomic nervous system disturbance is seen in 75% of persons with LEMS. Dry mouth, constipation, hazy vision, reduced sweating, and orthostatic hypotension—a drop in blood pressure upon standing—might all be symptoms of this, which could result in blackouts. Some claim to have a metallic taste in their mouths. On a neurological exam, the weakness seen with standard power testing is frequently not as severe as would be expected based on the symptoms. Strength improves even more with repeated testing, such as the “Lambert’s sign” phenomenon where power increases with repeated hand grip. Reflexes are usually slower when at rest.
reflex strength improves with muscle use. This is a defining quality of LEMS. There may be a slow pupillary light reflex.
Most LEMS patients with lung cancer do not initially exhibit cancer-related symptoms such as a cough, blood in the cough, or accidental weight loss. LEMS may be severe when connected to lung cancer.
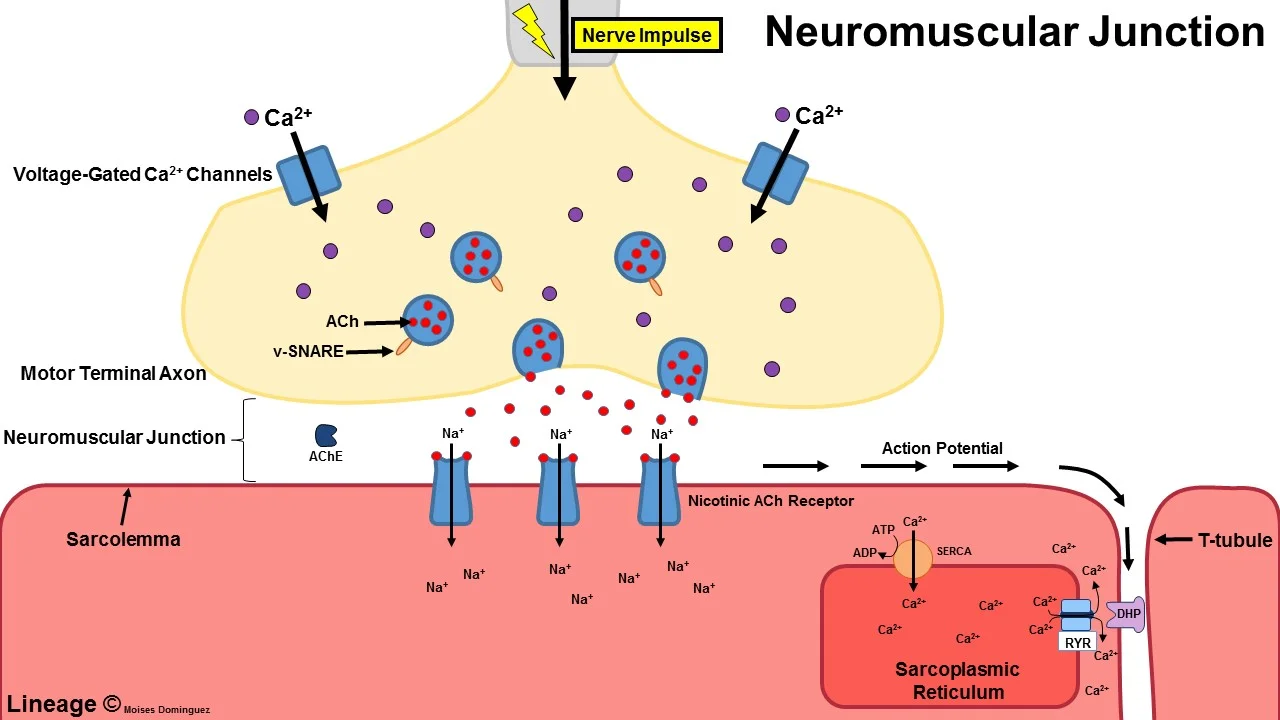
Mechanism
A nerve impulse comes from the spinal cord down the axon, the long projection of a nerve cell, to the muscles in proper neuromuscular function. At the nerve ending in the
The nerve impulse causes the opening of voltage-gated calcium channels (VGCC) at the neuromuscular junction, where the impulse is transmitted to the muscle cell.
calcium ion inflow and the calcium-dependent activation of synaptic vesicle fusion with the plasma membrane at the nerve terminal. Within these synaptic vesicles are
The cholinergic receptors on the muscle are stimulated by the release of acetylcholine into the synaptic cleft. Afterward, the muscle contracts.
Because fewer calcium ions may access the nerve endings in LEMS due to antibodies against VGCC, especially the P/Q-type VGCC, less acetylcholine can be produced.
from the neuromuscular junction again. Acetylcholine neurotransmission is necessary for the autonomic nervous system in addition to skeletal muscle, which explains why autonomic symptoms might appear in LEMS. The cerebellum also contains P/Q voltage-gated calcium channels, which explains why some people have coordination issues. The “domain III S5-S6 linker peptide” of the receptor is the area where the antibodies bind most strongly. Other VGCCs may also be bound by antibodies. The protein sensor for calcium-regulated vesicle fusion, synaptotagmin, is bound by antibodies in certain people. The existence of detectable antibodies against the M1 subtype of the acetylcholine receptor in many LEMS patients, including those with and without VGCC antibodies, may contribute to a lack of calcium influx compensation.
Along with the reduced calcium influx, LEMS patients also exhibit antibodies to the proteins found in these active zones, including the voltage-dependent calcium channels, which may contribute to the disruption of the active zone vesicle release sites. The reduction in muscular contractility is caused by these anomalies taken together. A study in electrodiagnostic medicine called needle electromyography has shown that repeated stimuli over a period of about 10 seconds eventually lead to sufficient calcium delivery and an increase in muscle contraction to normal levels by increasing the amplitude of repeated compound muscle action potentials.
It is believed that the antibodies first form as a reaction to the cancer cells because the antibodies discovered in LEMS linked to lung cancer also bind to calcium channels in the cancer cells. According to some theories, the immune response to cancer cells inhibits their proliferation and improves the prognosis.
Diagnosis
The medical professional will do a physical examination and go over your symptoms with you. You could be diagnosed with this illness through a particular blood test. A test called electromyography, which demonstrates how efficiently your muscles are operating, is another option you have. Your doctor could ask for X-rays or a CT scan of your lungs because Lambert-Eaton syndrome is linked to lung cancer. Lambert-Eaton syndrome has many symptoms with another disorder termed myasthenia gravis. To distinguish between the conditions, your healthcare professional will rely on electromyography and other test findings.
If your doctor determines you have this illness, they will evaluate you for lymphoma and other malignancies as well as lung cancer. Lambert-Eaton syndrome may manifest up to three years before a cancer diagnosis, therefore you may need to have regular checkups if cancer isn’t initially diagnosed.
The diagnosis of LEMS can be accurately confirmed by electrophysiological testing. While the CMAP is lower in nerve conduction investigations, the latencies and conduction velocities are normal. The best research to find LEMS is repetitive nerve stimulation (RNS). The CMAP will first decrease in a manner reminiscent of myasthenia gravis. On a brief workout, however, the CMAP will gradually increase. The duration of this is up to 30 seconds. The single-fiber EMG analysis is more sensitive, but it also calls for excellent technical skills. There will be more jitter, which denotes a disease at the neuromuscular junction. Regrettably, it cannot distinguish between MG and LEMS.
Most individuals with LEMS who have had their EMGs validated will have antibodies to voltage-gated calcium channels. The diagnosis may be confirmed by other laboratory testing, such as creatine kinase e and thyroid function tests.
When LEMS is definitively diagnosed, the doctor may advise a CT or MRI scan to check for tumors, particularly lung cancer.
History and Physical
Proximal muscular weakness, autonomic dysfunction, and absence of deep tendon reflexes are the three clinical signs of Lambert-Eaton myasthenic syndrome that are most frequently observed. In SCLC-LEMS, symptoms typically develop slowly and advance more quickly.
Muscle weakness: Patients report difficulties rising from a sitting posture since it often affects the proximal leg. The trend of advancement has been characterized as being proximal to distal, caudal to cranial, and eventually reaching the oculobulbar area. Muscle involvement is often symmetrical. Additionally, patients talk about a dull ache or stiffness. Examining the patient reveals areflexia without any obvious muscular atrophy.
With repeated muscular contractions, postexercise or post-activation facilitation—which is characterized by the restoration of tendon reflexes and muscle strength—occurs. This condition is linked to Lambert-Eaton myasthenic syndrome. If performed after a little time of patient rest, the exam will be more obvious.
Cranial nerve involvement may be present in up to 70% of individuals with Lambert-Eaton myasthenic syndrome, which causes oculobulbar paralysis. The most typical cranial nerve manifestations of Lambert-Eaton myasthenic syndrome are ocular symptoms, including ptosis and diplopia. Dysarthria and dysphagia are also seen. In the final stages of the disease, these symptoms frequently surface.
In 80% to 96% of patients, autonomic dysfunction is noted. The most frequent symptom mentioned is a dry mouth. Men’s erectile dysfunction, constipation, orthostatic dysfunction, and impaired mental status are further symptoms of perspiration.
In the final phases of a patient with Lambert-Eaton myasthenic syndrome, respiratory failure is uncommon.
Evaluation
Evaluation for Lambert-Eaton myasthenic syndrome should be prompted by the presence of clinical symptoms such as proximal muscle weakness coupled with areflexia and autonomic dysfunction. P/Q-type VGCC and electrodiagnostic tests can both be used to confirm the diagnosis of Lambert-Eaton myasthenic syndrome.
Serology
In between 85% and 95% of individuals with Lambert-Eaton myasthenic syndrome, antibodies against the P/Q-type VGCC were found in a radioimmunoassay. The existence of P/Q-type VGCC has been linked to a number of neurological illnesses and other autoimmune diseases, therefore it is not unique to Lambert-Eaton myasthenic syndrome. Antibodies against N-type VGCC are detected in Lambert-Eaton myasthenic syndrome less commonly (between 30% and 40%). Additionally, it was discovered that 64% of SCLC patients with Lambert-Eaton myasthenic syndrome also had antibodies against SOX1, a 95% specific immunogenic tumor antigen in SCLC.
Electrodiagnostic Testing
Eaton and Lambert first observed low CMAP (compound muscle action potential) amplitude at rest, a decreasing response to low rates of repeated nerve stimulation (RNS), and an increasing response to high rates of stimulation during electrodiagnostic testing. Although other results have sometimes been reported, these findings have mainly proven repeatable.
A large incremental response in CMAP amplitude is observed after high-frequency RNS or post-exercise, but only an increase of 60% to 99% is regarded as diagnostic.
Action potentials that are unstable can be seen on a needle EMG.
When firing at greater rates, single fiber electromyography (SFEMG) often exhibits less jitter and transmission-blocking. Compared to RNS, SFEMG is more sensitive. RNS, on the other hand, is more generally accessible and helpful in differentiating between Lambert-Eaton myasthenic syndrome and myasthenia graves by exhibiting post-exercise facilitation.
Screening for Malignancy
The diagnosis of Lambert-Eaton myasthenic syndrome should trigger an early and thorough search for underlying malignancy due to its close association with cancer. The first imaging test that is advised is either a CT or an MRI of the chest. If the CT scan is negative, the first screening also includes a PET scan. If the initial assessment results in a negative finding, cancer screening should continue every three to six months for at least two years. Patients at high risk for developing SCLC-LEMS who have a DELTA-P score of more than 2 or who have positive SOX antibodies should be screened every three months.
The Dutch-English LEMS Tumour Relationship Prediction (DELTA-P) score is used to risk stratify the relationship with SCLC in patients with Lambert-Eaton myasthenic syndrome and direct the screening for underlying malignancy. It includes factors including the age of diagnosis and smoking history.
Treatment of Lambert-Eaton Syndrome
Your healthcare professional may use surgery, radiation, or chemotherapy to treat your cancer. Your Lambert-Eaton syndrome is more likely to improve if you have cancer and respond well to therapy. Additionally, your doctor can recommend drugs to weaken your immune system or to enhance the communication between your nerve and muscle cells.
Additionally, you might receive a procedure called plasmapheresis. Your blood’s plasma must be replaced in order to do this. By doing this, toxic immune system proteins that may be contributing to the illness are removed from your blood.
Increasing acetylcholine levels is the primary goal of treatment for Lambert-Eaton myasthenic syndrome, whether it is associated with cancer or not. Weakness is treated with acetylcholinesterase inhibitors, such as pyridostigmine (30 to 120 mg every 3 to 6 hours). The effects, meanwhile, are not as obvious as they are in myasthenia gravis patients. 3,4 -aminopyridine (3,4- DAP) is an additional treatment option. When 3,4-DAP binds to VGCCs, it prolongs the action potential’s depolarization and lengthens their open times, which increases presynaptic calcium influx and ACh release.
First-line treatment for people with refractory weakness is immunosuppression with IVIG. Prednisone, rituximab, azathioprine, and plasma exchange are some further recommended substitutes.
Your healthcare professional may use surgery, radiation, or chemotherapy to treat your cancer. Your Lambert-Eaton syndrome is more likely to improve if you have cancer and respond well to therapy. Additionally, your doctor may suggest medications that will impair the communication between your nerve and muscle cells or suppress your immune system..
You might also undergo a technique called plasmapheresis. To accomplish this, you must replenish the plasma in your blood. Toxic immune system proteins that might be a factor in the illness are eliminated from your blood by doing this.
The therapy of the underlying malignancy is necessary for Lambert-Eaton myasthenic syndrome caused by SCLC.
Medical Management
Since there is still no “cure,” medical management is limited to treating symptoms. The following are some frequent medical procedures:
Pharmacotherapy
By reducing mitochondrial activity, potassium channel blockers like guanidine and aminopyridine raise intracellular calcium levels.
Acetylcholinesterase inhibitors, such as pyridostigmine, stop ACh from being broken down and permit it to build up in the synaptic cleft.. As a result, post-synaptic ACh receptors are frequently and constantly activated.
The associated tumor is the target of chemotherapy treatments.
Immunotherapy
Early in the course of the illness, immunomodulating therapies including plasmapheresis and intravenous immunoglobulin injection are successful.
The immunosuppressive effects of corticosteroid treatment may help some people.
Immunosuppression
The use of intravenous immunoglobulin (IVIG) is supported by some data. When compared to other autoimmune disorders, immune suppression is frequently less successful. Prednisolone, a glucocorticoid or steroid, lowers the immunological response; once the intended therapeutic effect has been achieved, azathioprine, a steroid-sparing drug, may be used in its place. IVIG has some potential for effectiveness. Acute extreme weakness may improve with plasma exchange (or plasmapheresis), which involves removing plasma proteins like antibodies and replacing them with normal plasma. Again, plasma exchange is less effective than in disorders like myasthenia gravis that are related to it, and extra immunosuppressive drugs are frequently required.
Other
Surgery
In some circumstances, the tumor may need to be surgically removed.
Physiotherapy Treatment
As of right now, no scientific trials have examined how well PT treats LEMS. Therefore, with caution, therapies that have been proven to be successful in myasthenia gravis may be used to the care of individuals with LEMS. The goal of symptomatic treatment is to maintain as much functional independence as possible.
Differential Diagnosis
Differential MG is a component of the Lambert-Eaton myasthenic syndrome diagnosis. Areflexia, autonomic dysfunction, and the phenomenon of post-exercise facilitation found in Lambert-Eaton myasthenic syndrome are the defining characteristics. The differential diagnosis of Lambert-Eaton myasthenic syndrome still includes myopathies. It is easier to rule out polyneuropathy or polyradiculopathies when there are no sensory complaints.
Enhancing Healthcare Team Outcomes
The diagnosis and management of patients with Lambert Eaton myasthenia syndrome require an interprofessional team because of the diverse presentation. The team should include of an oncologist, surgeon, hematologist, ophthalmologist, neurologist, primary care provider, and nurse practitioner because the disease is typically encountered in the presence of a malignancy. Increasing acetylcholine levels is the primary goal of treatment for Lambert-Eaton myasthenic syndrome symptoms, whether or not there is a tumor present. First-line treatment for people with refractory weakness is immunosuppression with IVIG. Prednisone, rituximab, azathioprine, and plasma exchange are some further recommended substitutes.
The primary malignancy affects the prognosis for people with this condition. The prognosis is bad for advanced instances. Even if clinical improvement does happen over time if the main tumor is under control, complete healing is frequently not achievable.
Prognosis
Assessing the prognosis is frequently challenging. It is mostly influenced by the kind of cancer that may be present in the background, the existence and severity of any autoimmune conditions that may be present, and the degree and distribution of weakening. Additionally, patients with symptoms that worsen quickly typically have more serious illnesses.
The main issue brought on by LEMS is the deteriorating strength that impairs daily life and overall quality of life. LEMS does not appear to have the same substantial effects on the respiratory system as MG. Most patients’ essential muscles are not badly affected by weakening. Within a few months of the commencement of symptoms, the maximum severity is typically determined.
Therapy with drugs such as 3,4-aminopyridine (DAP) may occasionally assist to partially ease symptoms, although symptoms typically worsen over time. Weakness and dysfunction rarely change without treatment. The only exceptions are times of aggravation brought on by a concurrent sickness or by drugs that interfere with neuromuscular transmission.
The weakness brought on by LEMS may eventually have significant repercussions. However, the underlying cancer frequently causes mortality. LEMS diagnoses frequently portend malignancy. Since SCLC has a very low survival period, this connection is crucial for total morbidity.
The prognosis of SCLC in patients with SCLC-LEMS is better than in SCLC without LEMS because LEMS may result in early identification of SCLC. A more potent immune response to the malignancy may be present in SCLC patients who develop LEMS, improving survival. Patients with SCLC-LEMS have a higher likelihood of experiencing a quicker clinical course.
If there is no evidence of underlying cancer and the LEMS has been present for at least two years, the condition was likely brought on by an autoimmune process. The prognosis at that stage is based on the degree of malfunction as well as the existence and severity of other autoimmune disorders.
Can Lambert-Eaton syndrome be prevented?
It’s not entirely obvious how to prevent Lambert-Eaton syndrome because the specific source of the condition is yet unknown. Avoiding smoking is the best method to lower your risk of lung cancer, which is frequently linked to Lambert-Eaton syndrome.
Other steps that could reduce your risk of developing lung cancer include:
Avoid exposure to tobacco smoke.
Eat more fruits and vegetables.
Have your home checked for radon.
Living with Lambert-Eaton syndrome
Lambert-Eaton Syndrome symptoms could get worse if you’re hot or contagious. As a result, refrain from taking hot baths or showers, and get in touch with your doctor as soon as you notice any symptoms of the flu or a cold. Regular exercise and sufficient rest may also assist you in controlling your symptoms..
Key points
The immune system of the body targets the connections between the muscles and nerves in Lambert-Eaton syndrome. Although it can happen in persons without cancer, it is most frequently observed in those with small-cell lung cancer or other tumors.
Weak muscles, difficulty walking, tingling, exhaustion, and dry mouth are typical symptoms.
Finding and treating any underlying cancer is the top goal because doing so may help with the condition’s symptoms. The use of medications to weaken the communication between the nerves and muscles or to inhibit the immune system are examples of further treatments.
FAQs
How long can someone tolerate LEMS?
Patients with LEMS and SCLC typically have relatively bad prognoses since SCLC (see this word) is a particularly aggressive malignancy. Although the median survival time is 17 to 24 months, 20% of patients have long-term remissions or are cured (compared to 2% of individuals with SCLC who do not have LEMS).
Lambert-Eaton hypersensitivity is of what kind?
Type II hypersensitivity responses encompass a large number of immunological disorders mediated by antibodies. An illness caused by immunologic antibodies is Lambert-Eaton syndrome. The first broad classification of disease it belongs within is autoimmune disorders since the antibodies are directed against a self-antigen.
Which drugs need to be avoided if you have Lambert-Eaton myasthenic syndrome?
Some antibiotics have strong neuromuscular blocking effects, including aminoglycosides, fluoroquinolones (like ciprofloxacin), and erythromycin. Some beta-adrenergic blockers and antiarrhythmic medications, such as quinine, quinidine, and procainamide, exacerbate myasthenic weakness.
What does Lambert Eaton’s repeated nerve stimulation entail?
Workup for Lambert-Eaton Myasthenic Syndrome (LEMS): Method…
By exhibiting distinctive findings, repetitive nerve stimulation (RNS) investigations support the diagnosis of LEMS (see the illustration below). Compound muscle action potentials (CMAPs) are typically tiny, frequently less than 10% of normal, and fall between 1 and 5 Hz during RNS.
LEMS: Does it lead to neuropathy?
In Lambert-Eaton myasthenic syndrome, an autoantibody is directed against the pre-synaptic calcium channel, resulting in a disruption of neuromuscular transmission. It manifests as increased leg weakness in the proximal limbs, together with or without peripheral and autonomic neuropathy.