Macrocephaly
What is Macrocephaly?
The term “macrocephaly” refers to an enlarged head size. If your infant has macrocephaly, it means their head circumference, measured around the widest part of their head, is significantly larger than that of other children of the same age and sex. Technically, their head circumference falls above the 97th percentile, indicating that their head is larger than that of 97% of children in the same age and sex group.
Macrocephaly can serve as an indicator of an underlying condition that may require medical intervention. However, it’s important to note that a larger head size in your baby could also be a hereditary trait that runs in your family, posing no harm and not necessitating treatment. This specific condition is known as “benign familial macrocephaly
Introduction
The measurement of head circumference, often referred to as OFC (Occipital Frontal Circumference), serves as a valuable tool for tracking and monitoring childhood growth and development. On average, during the first three months of life, head circumference increases by approximately 2 cm per month, followed by a rate of 1 cm per month from 3 to 6 months of age. In the latter half of infancy, there is a slower growth rate, with a gain of approximately 0.5 cm per month. Over the course of the first year, there is an average increase of 12 cm in head circumference. Beyond the first year, the growth rate slows further, with only a 1 cm gain every six months until the age of three. From ages 3 to 5, there is an annual increase of 1 cm, resulting in a total gain of approximately 5 cm in head circumference between 1 to 5 years of age.
Accurate measurement of head circumference can pose challenges, especially with restless young infants and in cases with thick hair. It is crucial to ensure the proper placement of the measuring tape on anatomical landmarks. Serial measurements of head circumference should be conducted during each health supervision visit up to 24 to 36 months of age to assess head growth velocity. Vigilant monitoring of head size is particularly critical in high-risk situations, such as with preterm infants and those affected by conditions like bacterial meningitis, subdural hematoma, and hydrocephalus. Whenever abnormal readings are encountered, it is essential to double-check them for accuracy.
Macrocephaly is characterized by a head circumference that exceeds two standard deviations above the mean for gestational age and sex, placing it above the 97th percentile. It’s important to note that, despite often being used interchangeably, megalencephaly is a distinct term denoting increased growth of cerebral structures. Macrocephaly, on the other hand, encompasses a broader range of causes for increased head size, including those not associated with cerebral overgrowth, such as subdural fluid collection.
Understanding the various etiologies of macrocephaly is crucial. This knowledge helps differentiate benign macrocephaly from conditions that demand immediate investigation and intervention to prevent potential long-term neurological deficits and developmental delays.
Causes of Macrocephaly
Macrocephaly results from an enlargement in any of the components within the cranium, which includes the brain, cerebrospinal fluid (CSF), blood, or bone. Alternatively, it can be associated with increased intracranial pressure (ICP). The underlying causes of macrocephaly tend to vary based on the age at which it becomes evident, with familial megalencephaly being the most common cause, especially in cases of mild macrocephaly with normal development.
Increased Brain Parenchyma:
Anatomic Megalencephaly: This form arises from an increase in the size or number of brain cells without concurrent metabolic disease or acute encephalopathy. Typically, it is present at birth, with the occipitofrontal circumference (OFC) continuing to increase along a trajectory parallel to the upper growth curve percentiles postnatally.
- Familial Megalencephaly: Benign familial megalencephaly, the most common type of anatomic megalencephaly, presents with children born with large heads and normal body size. During infancy, the OFC exceeds the 90th percentile, often by 2 to 4 cm (0.8 to 1.5 inches), while remaining parallel to the 98th percentile. The OFC may increase at a rate of 0.6 to 1 cm (0.2 to 0.4 inches) per week, compared to the usual 0.4 cm (0.15 inches) per week. Head growth velocity normalizes by around six months of age. In cases of normal neurologic exams, typical development, an absence of syndrome-specific clinical features, and no family history of neurologic or developmental issues, familial megalencephaly can be confirmed by comparing the child’s OFC to their parents’ head circumferences using Weaver curves. If the child’s OFC falls within the normal ranges indicated by the Weaver curves, neuroimaging is unnecessary.
- Other Causes of Anatomic Megalencephaly: These include neurocutaneous disorders (e.g., neurofibromatosis, tuberous sclerosis complex, linear sebaceous nevus syndrome, and hypomelanosis of Ito), autism spectrum disorder (ASD), achondroplasia, cerebral gigantism (Sotos syndrome), fragile X syndrome, Cowden syndrome, and nevoid basal cell carcinoma syndrome (Gorlin syndrome).
Metabolic Megalencephaly:
Metabolic megalencephaly results from the deposition of metabolic products in brain tissue. In these cases, the OFC in children is generally within the normal range at birth but increases during the neonatal period. Diseases causing metabolic megalencephaly include leukodystrophies (e.g., Alexander disease, Canavan disease, megalencephalic leukoencephalopathy), lysosomal storage disorders (e.g., Tay-Sachs disease, mucopolysaccharidosis, and gangliosidosis), and organic acid disorders (e.g., glutaric aciduria type 1).
Increased Cerebrospinal Fluid:
Hydrocephalus: Hydrocephalus is characterized by excessive CSF in the cerebral ventricular system, leading to increased pressure and dilatation. It can be caused by increased production, reduced absorption, or CSF flow obstruction. Increased OFC is often the initial sign of hydrocephalus. It’s essential to note that benign enlargement of the subarachnoid space can also lead to macrocephaly.
Increased Blood:
Increased intracranial blood volume may arise from hemorrhage (intraventricular, subdural, epidural) or arteriovenous malformations. However, macrocephaly is rarely the sole manifestation of intracranial hemorrhage.
Increased Bone:
Bone thickening causing macrocephaly can result from factors like bone marrow expansion (seen in thalassemia major) or primary bone disorders (e.g., skeletal and cranial dysplasias).
Increased Intracranial Pressure:
Increased ICP may be idiopathic (pseudotumor cerebri) or caused by various factors such as increased intracranial contents (brain, CSF, blood), mass lesions, infection, inflammation, or metabolic abnormalities.
Mass Lesions:
Intracranial cysts, tumors, or abscesses are examples of mass lesions. However, increased OFC is rarely the only symptom of these conditions.
In summary, macrocephaly can be attributed to a wide range of causes, necessitating careful evaluation and diagnosis to determine appropriate treatment or monitoring.
Epidemiology of Macrocephaly
Macrocephaly, by definition, is observed in approximately 2% to 3% of the population, with no significant gender-based variations. The epidemiological details, including geographic distribution, are contingent on the underlying causes contributing to this condition.
Pathophysiology of Macrocephaly
The development of macrocephaly is intricately tied to its specific underlying causes, with each etiology contributing to the condition through distinct mechanisms. It may stem from the excessive growth of the skull bones or an enlargement of intracranial structures, including the cerebrospinal fluid (CSF), blood, or brain parenchyma. For a comprehensive understanding of the factors linked to macrocephaly, a detailed review of the etiology section is recommended.
Symptoms of Macrocephaly
If your infant displays specific physical or behavioral symptoms, it can understandably raise concerns, prompting a visit to the doctor. These concerns may include:
- Tightness or bulging of the soft spot (fontanelle)
- Unusual eye movements
- Unexplained projectile vomiting
- Increasing irritability over time
- A high-pitched cry that seems indicative of pain or discomfort
- Developmental delays
When these worrisome signs are present, healthcare professionals will undertake a thorough assessment to determine the root cause of the head size variation. They typically seek to ascertain whether the enlarged head is a result of factors such as an enlarged brain, excessive fluid accumulation within the skull, or an overgrowth of the skull bones. To arrive at a conclusive diagnosis, imaging tests like MRI (Magnetic Resonance Imaging) or CT (Computed Tomography) scans are employed. In some cases, further investigations, such as blood tests or genetic testing, may be necessary to reach a definitive diagnosis. This comprehensive evaluation aids in identifying and addressing any underlying issues promptly and effectively.
Macrocephaly Due to Hydrocephalus
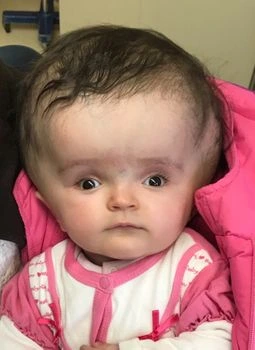
Hydrocephalus, often referred to as ‘water on the brain,’ is characterized by an accumulation of excess cerebrospinal fluid around the brain. In some instances, particularly when it affects infants, it is considered benign and goes by names such as ‘benign extra-axial collections of infancy’ or ‘benign external hydrocephalus.’ Typically, children naturally outgrow this condition during early childhood. Nonetheless, regular follow-up with a healthcare professional is essential to monitor your baby’s progress.
However, there are situations where hydrocephalus can pose serious risks. The increased fluid pressure can lead to potential brain injuries, necessitating surgical intervention to alleviate the pressure and reduce the risk of long-term brain damage.
Genetic Condition Associated With Macrocephaly
Occasionally, macrocephaly serves as a symptom of an underlying genetic condition. Several such conditions encompass:
- Sotos Syndrome: This disorder triggers rapid physical growth both before birth and during the initial years of life. In addition to macrocephaly, individuals with Sotos syndrome often exhibit an elongated head shape and may experience developmental delays.
- Cowden Syndrome: Cowden syndrome manifests as non-cancerous growths on a person’s body, potentially indicating a heightened risk of certain cancers later in life.
- Gorlin Syndrome (Basal Cell Nevus Syndrome): Gorlin syndrome presents with an enlarged head, distinctive facial features, and pitted skin on the hands and feet. It also leads to benign tumor growth and elevates the risk of basal cell skin cancer in young adults.
- Fragile X Syndrome: Fragile X syndrome arises due to the body’s inability to produce a specific protein crucial for brain development. It results in developmental delays, learning, and intellectual disabilities, as well as social-emotional challenges.
It’s important to note that genetic conditions like these lack a cure. Instead, your doctor will collaborate with you to manage your child’s symptoms effectively. This comprehensive approach may encompass medical treatments and various therapies tailored to address physical and developmental delays, ensuring a personalized plan to meet your child’s unique needs.
How is Macrocephaly Diagnosed?
Macrocephaly is typically diagnosed using a growth chart. During your child’s routine checkups, until they reach 3 years of age, their healthcare provider will measure their head circumference.
Here’s how the diagnosis process usually unfolds:
- Confirming Measurements: Infants can be quite active during measurements, so if the head circumference seems unusual, your child’s healthcare provider will take additional measurements to ensure accuracy.
- Parental Measurements: If macrocephaly is confirmed, the healthcare provider will also measure the head circumference of both parents. The Weaver Curve is a useful tool that can help determine if your child’s head size is largely due to genetic factors.
- Ultrasound: If benign familial macrocephaly is ruled out, the next step is usually a head ultrasound. This ultrasound can reveal whether your baby has benign extra-axial fluid collections or if there are indications of more serious conditions such as hydrocephalus or a tumor. Research shows that ultrasounds are highly reliable for diagnosing macrocephaly and can effectively rule out severe conditions. In fact, only about 5% of infants require a CT scan or MRI following an ultrasound. Ultrasounds are preferred for their safety, as they do not involve radiation and are quick and painless.
- CT Scan or MRI: If the ultrasound reveals concerning findings or if your child’s fontanelle (soft spot) has already closed, the healthcare provider may recommend a head CT scan or MRI. MRI is often preferred for infants as it does not involve radiation. These advanced imaging studies can provide more detailed information and help identify rare causes of macrocephaly such as hydrocephalus, tumors, or bleeding.
- Developmental Monitoring: While imaging studies can identify structural issues, they cannot diagnose conditions like autism. Therefore, your healthcare provider will also closely monitor your child’s development for any signs of medical conditions associated with macrocephaly. They may recommend consulting with a developmental pediatrician or geneticist for a more comprehensive assessment.
This diagnostic process ensures that any underlying causes of macrocephaly are accurately identified and addressed, allowing for appropriate management and care tailored to your child’s needs.
Approach to Macrocephaly by Compartment
Enlarged Extraaxial Spaces:
Benign enlargement of subarachnoid spaces is a common cause of macrocephaly in infants, affecting up to 75% of cases. Infants typically present with a large head and normal to mildly delayed cognitive development. This condition often resolves naturally by the age of 2 and may be linked to immature or impaired cerebrospinal fluid (CSF) reabsorption at the arachnoid granulation.
Imaging reveals symmetric enlargement of subarachnoid spaces along the frontoparietal convexities and widening of the interhemispheric sulcus. Mild supratentorial ventriculomegaly may also be observed. Bridging veins crossing the subarachnoid space appear as elongated, nonstretched flow voids on T2-weighted images. These veins can also be visualized using Doppler ultrasound. While benign enlargement of subarachnoid spaces has been associated with isolated subdural hemorrhage (SDH), caution is advised when diagnosing it in children presenting to the emergency department. Notably, prominent subarachnoid spaces and ventricles in a child with microcephaly or a normal-sized head should raise concerns about brain parenchymal volume loss rather than benign subarachnoid space enlargement.
Abusive Head Trauma (AHT):
AHT, a significant cause of long-term morbidity and mortality in children, often occurs during the first year of life. SDH is a common finding in AHT and can increase head circumference, particularly with repeated traumatic events from a young age. SDH results from injury to bridging veins due to various forces. The combination of SDH, retinal hemorrhage, and neurological dysfunction is highly indicative of AHT, also known as shaken-baby syndrome.
Imaging typically shows supratentorial SDH as crescent-shaped collections along the convexity, the posterior falx, or the tentorium. Repeated hemorrhages can lead to layered collections with varying signal intensity or attenuation reflecting different ages of blood. Subdural hygromas may form due to arachnoid lacerations allowing CSF to migrate into the subdural space. This can lead to a combination of hematoma and hygroma. Diagnosing the age of subdural collections can be challenging.
Veins within the subarachnoid space may abut the brain parenchyma and become compressed by the subdural collection. Bridging vein thrombosis commonly occurs near the vertex and is best visualized on coronal images. Specialized sequences like T2*-weighted gradient-recalled echo and susceptibility-weighted imaging may show a round area of decreased signal intensity along a stretched bridging vein, indicative of clot formation. Additional signs of abusive trauma include retinal hemorrhage (best seen on susceptibility-weighted images), cerebral contusions and lacerations, abnormal suture diastasis, diffuse axonal injuries, and multiple fractures.
Glutaric Aciduria Type 1:
Glutaric aciduria type 1 is a rare autosomal recessive neurometabolic disorder resulting from a deficiency in glutaryl–coenzyme A dehydrogenase. This condition leads to the accumulation of glutaric acids, causing progressive hypotonia and neurologic deterioration. Macrocephaly is a key characteristic, typically present at birth or shortly afterward. Megalencephaly can also occur in this condition.
Imaging features often include widened sylvian fissures with open opercula and enlarged CSF spaces anterior to the temporal lobes, accompanied by white matter edema. Spontaneous SDH is estimated to occur in approximately 20–30% of patients with glutaric aciduria type 1, potentially leading to misdiagnosis as AHT. Therefore, when macrocephaly is associated with the classic imaging features, glutaric aciduria type 1 should be strongly considered as the underlying cause.
Enlarged Ventricles (Hydrocephalus):
Hydrocephalus, characterized by ventricular enlargement, presents with macrocephaly when onset occurs before 2 years of age. It can be classified as intraventricular (obstructive) or extraventricular (communicating). Intraventricular hydrocephalus typically results from impaired CSF flow through the foramen of Monro, Luschka, or Magendie, often secondary to mass lesions or intraventricular hemorrhage in premature infants. Extraventricular hydrocephalus arises from impaired CSF reabsorption at the level of the arachnoid granulations, often due to chronic accumulation of blood products or inflammatory scarring.
Common imaging findings in hydrocephalus include downward bowing of the third ventricular floor, enlargement of anterior and posterior recesses, temporal horn dilatation, effacement of cerebral sulci, periventricular interstitial edema, thinning of the corpus callosum, and sometimes ventricular diverticula or pseudodiverticula. Children with hydrocephalus leading to macrocephaly may also exhibit frontal bossing, thin skulls with gyral markings, suture diastasis, and prominent diploic veins.
Congenital Hydrocephalus:
Aqueductal stenosis is a common cause of congenital hydrocephalus, accounting for approximately 20% of cases. It is typically associated with macrocephaly. Focal stenosis often occurs at the level of the superior colliculi or the intercollicular sulcus. In some cases, aqueductal stenosis can be caused by an aqueductal web, thin membrane, or extrinsic compression, such as by an arachnoid or choroid plexus cyst.
Imaging shows supratentorial ventricular dilatation with inferior bulging of the third ventricular floor and a normal fourth ventricle. High-resolution cisternographic images can help determine the exact site of narrowing or web. The degree of stenosis may vary, with proximal stenosis often resulting in more severe hydrocephalus. Funneling of the superior portion of the aqueduct and multiple anastomotic canaliculi may also be present. Other brain malformations, such as rhombencephalon synapsis, can be associated with aqueductal stenosis.
Chiari Malformation Type 2:
Chiari malformation type 2 is the most familiar syndromic reason of hydrocephalus. It is characterized by a range of encephalic and skull base abnormalities. Classic imaging findings include inferior displacement of the cerebellar tonsils and vermis through the foramen magnum into the cervical canal, with the medulla and fourth ventricle often displaced inferiorly as well. Hydrocephalus is commonly associated with Chiari malformation type 2 and contributes to macrocephaly.
Patients with Chiari malformation type 2 often have a small posterior fossa with upward displacement of the tentorium. An enlarged fourth ventricle may also be present. Other associated findings include myelomeningocele and syringomyelia. If Chiari malformation type 2 is suspected in a fetus, maternal-fetal MRI can be performed to evaluate the hindbrain and skull base.
Infantile Neuronal Ceroid Lipofuscinosis:
Infantile neuronal ceroid lipofuscinosis is a rare, autosomal recessive lysosomal storage disorder. It primarily affects the central nervous system, leading to progressive neurologic deterioration. Megalencephaly is often present at birth and is a key characteristic.
Imaging typically reveals cortical and subcortical atrophy with white matter involvement. The cerebral cortex may appear abnormally thickened and show abnormal gyral patterns. Abnormal accumulation of lip pigments can result in hyperintense signal changes in the basal ganglia, thalami, and other brain regions on T2-weighted images. Progressive cerebral atrophy with ventriculomegaly may occur over time.
Evaluation of Postnatal Macrocephaly
Macrocephaly in children, characterized by an abnormally large head circumference, warrants careful evaluation. This assessment is prompted by various factors, including:
- Abnormal Head Circumference Measurement: When a single measurement of the occipitofrontal circumference (OFC) is abnormal or when serial measurements show a progressive enlargement. For infants younger than 6 months, an OFC increase exceeding 2 cm (0.8 inches) per month is concerning.
- Verification of Measurement: It’s essential to verify the measurement, as isolated abnormal readings may result from technical errors.
The assessment process involves a comprehensive history and physical examination of the child and parents, considering the potential familial variation in head size. Further evaluation, such as neuroimaging and additional tests, should be guided by clinical findings from the history and examination.
Factors Increasing Urgency and Extent of Evaluation:
Several factors necessitate more urgent and extensive evaluation, including:
- Open Fontanelles: If the fontanelles remain open, they require prompt attention.
- History of CNS Trauma or Infection: A history of central nervous system (CNS) trauma or infection, which may raise concerns regarding increased intracranial pressure or other neurological issues.
- Associated Symptoms: Symptoms like headache, ataxia, neurodevelopmental abnormalities, or syndromic features demand a thorough evaluation.
- Family History: A family history of neurologic or cutaneous abnormalities, genetic disorders, or malignancies can be significant.
Elevated ICP, CNS Trauma, or CNS Infection:
Urgent evaluation is imperative if the child exhibits symptoms or signs of increased intracranial pressure (ICP), CNS trauma, or CNS infection, including headache, vomiting, altered mental status, bulging fontanelle, or papilledema.
Syndromic Features:
When a child displays syndromic features, consultation with a clinical geneticist is warranted to determine the appropriate diagnostic evaluation.
Developmental Delay:
Children with developmental delay, in the absence of syndromic features, should undergo neuroimaging, typically with MRI or CT. This imaging may reveal underlying causes like hydrocephalus, leukodystrophy, or specific brain lesions.
Normal Development:
If a child’s macrocephaly is modest, development is normal, and syndromic features are absent, measuring the OFC of first-degree relatives can help assess for familial macrocephaly. In infants with open fontanelles, ultrasonography of the head can be considered.
History and Examination:
When assessing macrocephaly, a comprehensive history and examination are crucial. Key aspects include:
- History: Birth measurements, growth trajectory, seizure history, and any predisposing factors for hydrocephalus or other neurological issues should be obtained. Family history of relevant conditions must be explored.
- Physical Examination: The physical examination should focus on assessing general appearance, dysmorphic features, OFC measurement, weight and stature trajectories, fontanelle status, eye examination for papilledema or retinal abnormalities, skin examination for cutaneous findings, cardiovascular review for congenital heart disease, abdominal examination for hepatosplenomegaly, and musculoskeletal examination for signs of skeletal dysplasia.
Assessment of Familial Macrocephaly:
To assess familial macrocephaly, OFC measurements of parents should be obtained. The Weaver curve can help determine the genetic contribution to macrocephaly by calculating standard scores (SS) for the child and parents. If the child’s SS falls within the range determined by the average parental score, genetic influences on macrocephaly are suggested.
Neuroimaging:
Neuroimaging is a critical component of the evaluation process. The choice between radiographs, ultrasonography, CT, or MRI depends on factors like age, suspected etiology, symptoms, and availability. MRI is generally preferred over CT due to its avoidance of ionizing radiation. In infants with open fontanelles, head ultrasonography can be non-invasive and valuable. Consultation with pediatric neurologists or neuroradiologists can aid in selecting the most appropriate imaging modality.
Other Diagnostic Tests:
Additional diagnostic tests are guided by clinical findings. Children with syndromic macrocephaly may require evaluation for associated abnormalities, metabolic assessments, genetic studies, or electroencephalograms. Referral to specialists, such as clinical geneticists or pediatric neurologists, may be necessary based on the evaluation results.
Evaluation of Prenatal Macrocephaly
Prenatal macrocephaly is typically identified through ultrasound examinations. It is defined as a head circumference exceeding 2 standard deviations above the mean or surpassing the 98th percentile for gestational age, based on either the last menstrual period or femur length. However, diagnosing prenatal macrocephaly comes with challenges due to the limitations in measuring head circumference accurately and inconsistencies between prenatal and postnatal head circumference growth curves. Although there are reference values for fetal head circumference, specific standards tailored to various populations, such as sex or race/ethnicity, have not been established.
The approach to evaluating prenatal macrocephaly varies depending on several factors, including associated ultrasonographic anomalies, the appropriateness of other fetal biometric parameters (like bone length and abdominal circumference) concerning gestational age, the patient’s medical history (e.g., consanguinity or familial macrocephaly), and the head circumference measurements of parents and siblings.
- Ultrasonographic Anomalies: The presence of associated ultrasonographic anomalies, such as callosal dysgenesis, malformations of cortical development, hypertelorism, enlarged kidneys, polydactyly, or hypoplastic long bones, may suggest syndromic microcephaly.
- Overgrowth Syndrome: If head circumference, abdominal circumference, and long-bone length are greater than expected for gestational age, it could indicate an overgrowth syndrome, like Sotos syndrome or Weaver syndrome.
- Familial Macrocephaly: In cases where fetal head circumference falls between 2 and 2.5 standard deviations above the mean for gestational age, and there are family members with macrocephaly but no signs of autosomal dominant conditions associated with macrocephaly, this might indicate familial macrocephaly. However, it’s unusual for this to present prenatally.
Additional evaluation, such as karyotyping or fetal brain MRI, may be sought if a specific diagnosis is desired to assist with pregnancy management. Indications for these evaluations could include parental consanguinity, family members with macrocephaly and characteristics of autosomal dominant conditions involving macrocephaly, or unexplained fetal macrocephaly when family members have normal head circumferences and fetal biometric parameters other than head circumference are appropriate for gestational age.
The developmental outcome of prenatal macrocephaly depends on the underlying cause and any associated abnormalities.
- Cesarean Delivery: In cases where the head circumference is significantly increased, and vaginal delivery is deemed impractical, cesarean delivery may be indicated. The specific criteria for recommending cesarean delivery can vary depending on factors such as gestational age at delivery, absolute and relative head circumference, and maternal pelvic size. Typically, when the head circumference exceeds 40 cm (16 inches), consideration should be given to an abdominal delivery.
The evaluation of prenatal macrocephaly is a complex process that considers various factors, including associated anomalies, overgrowth syndromes, familial history, and the need for further diagnostic tests. The decision for cesarean delivery should be made carefully, taking into account multiple factors to ensure the safety of both the mother and the baby.
Management of Macrocephaly
The approach to managing macrocephaly is contingent on its underlying cause. Below are the management strategies for various scenarios:
- Benign Domestic Macrocephaly & Benign Enlargement of the Subarachnoid Spaces in Infancy (BESSI ):
- If the child exhibits no neurological symptoms, is achieving developmental milestones, and has a family history of larger head size, it is likely that the enlarged head size is hereditary and runs in the family. In such circumstances, no precise therapy is required.
- BESSI is also considered a benign condition. It involves the presence of excess cerebrospinal fluid in certain brain areas, but this does not pose harm, and it typically resolves on its own without requiring treatment.
- Macrocephaly Linked to Genetic Causes:
- In situations where macrocephaly is associated with genetic causes and may result in developmental challenges or neurological issues, a multidisciplinary approach to management is often beneficial. This can include:
- Physical Therapy: Physical therapists can help address motor skill and mobility challenges that may arise due to macrocephaly.
- Occupational Therapy: Occupational therapists assist in developing fine motor skills, sensory processing, and activities of daily living.
- Speech and Language Therapy: Speech therapists work on speech and communication difficulties that may be associated with macrocephaly.
- Behavioral Therapy: Behavioral therapists can provide strategies for managing any behavioral issues or developmental delays.
- In situations where macrocephaly is associated with genetic causes and may result in developmental challenges or neurological issues, a multidisciplinary approach to management is often beneficial. This can include:
- Brain Bleeds/Fluid Buildup in the Brain:
- When macrocephaly is a result of conditions such as brain bleeds or fluid accumulation in the brain, surgical intervention may be necessary to alleviate the fluid buildup or stop the bleeding. The specific surgical procedure and follow-up care will depend on the individual case and its severity.
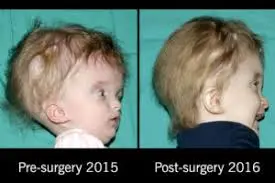
- Brain Tumor:
- If macrocephaly is linked to the presence of a brain tumor, the treatment approach typically involves a combination of medical interventions:
- Surgery: Surgery is often performed to remove the tumor and relieve any pressure it may exert on the brain.
- Chemotherapy: Chemotherapy may be recommended as part of the treatment plan to target and reduce the tumor’s size or prevent its recurrence.
- Radiation Therapy: Radiation therapy is sometimes employed to target and destroy cancerous cells in the brain.
- Steroids: Steroids may be prescribed to reduce inflammation and manage symptoms associated with the tumor.
- In all cases, the management of macrocephaly should be individualized based on the underlying cause, the child’s specific needs, and input from a team of healthcare professionals, including pediatricians, neurologists, and therapists. The goal is to optimize the child’s development and well-being while addressing any medical issues associated with macrocephaly.
- If macrocephaly is linked to the presence of a brain tumor, the treatment approach typically involves a combination of medical interventions:
Summary and Recommendations for Managing Macrocephaly
Monitoring head growth is a crucial aspect of pediatric care, particularly during the early years of life. Here, we provide a summary of key points and recommendations for the evaluation and management of macrocephaly:
- Monitoring Head Growth:
- Head circumference, also known as occipitofrontal circumference (OFC), should be routinely measured at health maintenance visits from birth to three years of age.
- Tracking OFC measurements over time provides valuable information for assessing growth trends and detecting potential issues.
- Definitions:
- Macrocephaly is defined as an OFC greater than two standard deviations (SD) above the mean for a specific age, sex, and gestational age, equivalent to being at or above the 97th percentile.
- Megalencephaly refers to the enlargement of brain parenchyma.
- Etiology:
- Macrocephaly can result from the enlargement of any component within the cranium, including the brain, cerebrospinal fluid, blood, or bone, or from increased intracranial pressure.
- Evaluation:
- The evaluation for macrocephaly should be initiated under the following circumstances:
- An individual OFC measurement is abnormal, following confirmation of accurate measurement techniques.
- Serial measurements demonstrate progressive enlargement.
- In infants younger than six months, the OFC increases by more than 2 cm per month (equivalent to 0.8 inches per month).
- Factors Influencing Evaluation Urgency:
- Several factors increase the urgency and scope of the evaluation:
- A history of central nervous system (CNS) trauma/infection.
- Presence of associated symptoms, neurodevelopmental abnormalities, or syndromic features.
- A family history of relevant neurological conditions.
- Neuroimaging:
- Neuroimaging, such as MRI or CT scans, is essential for children suspected of having an expanding cranial lesion.
- In cases where developmental delays are present but features suggestive of a particular syndrome are lacking, neuroimaging can aid in determining the underlying cause.
- The choice of imaging should prioritize the detection of significant intracranial pathology while minimizing potential risks associated with radiation exposure or sedation.
- Additional Diagnostic Evaluation:
- The history and physical examination guide further diagnostic evaluation.
- Consultation with a clinical geneticist may be valuable in determining the most appropriate diagnostic studies, especially in cases with syndromic features or complex familial histories.
In conclusion, the evaluation and management of macrocephaly demand a thorough and systematic approach. Consistent monitoring of head growth, careful assessment of clinical features, and appropriate diagnostic studies are essential for achieving accurate diagnoses and providing optimal care for affected children. Collaboration with specialists, including clinical geneticists, can be instrumental in tailoring diagnostic strategies to individual cases.
FAQ
What is the cause of macrocephaly?
The pathogenesis of macrocephaly is etiology-specific since it can be brought on by an expansion of the skull bones or by an expansion of the CSF, blood, or brain parenchyma, among other intracranial tissues.
Is macrocephaly a birth defect?
The heads of newborns with macrocephaly are disproportionately big. In comparison to 97% of newborns of the same age and sex, the circumference will be greater. A doctor will keep an eye on the baby because there might occasionally be an underlying issue. This disorder is frequently benign or unharmful.
What are the early symptoms of macrocephaly?
Parents should be on the lookout for symptoms of increased head pressure, such as a bulging fontanel (a soft region on top of the head), vomiting, poor eating, odd eye or limb movements, irritability or lethargy, and poor mental development.
Is macrocephaly a neurological disorder?
Neurodevelopment will be evaluated even if benign and familial macrocephaly do not lead to neurological problems. Developmental delay, epilepsy, and moderate hypotonia are transient signs of benign and familial macrocephaly, despite the absence of neurological diseases.
How is macrocephaly treated?
Depending on the cause of macrocephaly, there are several treatment options. Children with benign familial macrocephaly are often asymptomatic and do not require treatment. In these circumstances, periodic head size measurement is adequate, combined with ongoing physical and neurological development monitoring.
At what age is macrocephaly diagnosed?
Macrocephaly can be brought on by genetic conditions, or other illnesses, or it might run in families. A diagnosis can be made either before or after delivery by doing a head circumference measurement. To check for brain abnormalities and to hunt for a reason, doctors typically use genetic testing in addition to imaging tests.
Reference
- Jones, S. G. (2023, July 24). Macrocephaly. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560786/
- Fletcher, J. (2023, February 15). What to know about macrocephaly. https://www.medicalnewstoday.com/articles/322473#summary
- What You Should Know About Macrocephaly. (2021, April 23). WebMD. https://www.webmd.com/brain/what-you-should-know-about-macrocephaly
- UpToDate. (n.d.). UpToDate. https://www.uptodate.com/contents/macrocephaly-in-infants-and-children-etiology-and-evaluation#H482317963
- Bhavsar, K. (2023, March 27). What Is Macrocephaly. https://www.icliniq.com/articles/genetic-disorders/macrocephaly