Chiari Malformation
What is Chiari Malformation?
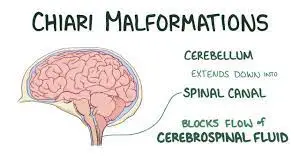
Chiari malformation (pronounced kee-AH-ree mal-for-MAY-shun) is a medical condition characterized by the displacement of brain tissue into the spinal canal. This condition arises when a portion of the skull is misshapen or smaller than usual, causing pressure on the brain and its downward displacement.
Although Chiari malformation is relatively uncommon, the increased use of diagnostic imaging techniques has led to more frequent diagnoses.
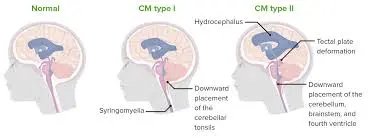
Doctors classify Chiari malformation into three primary types based on the specific anatomy of the displaced brain tissue and the presence of developmental issues in the brain or spine.
Chiari malformation type 1 typically develops during the growth of the skull and brain. Consequently, its signs and symptoms may not manifest until late childhood or adulthood. On the other hand, Chiari malformation types 2 and 3 are congenital, meaning they are present at birth.
The treatment of Chiari malformation depends on factors such as the type, severity, and associated symptoms. Treatment options may include regular monitoring, medications, or surgical intervention. In some examples, no remedy may be essential.
Chiari malformations encompass a diverse group of disorders characterized by structural anomalies in the cerebellum, brainstem, and the junction between the skull and the upper spine. These abnormalities result in the downward displacement of the cerebellum, either alone or in conjunction with the lower medulla, into the spinal canal.
This overview will delve into the anatomical and clinical aspects of the various Chiari malformation types.
Anatomical aspect related to Chiari malformation
Description: Chiari malformations are congenital conditions characterized by anatomical anomalies at the craniocervical junction, resulting in the downward displacement of cerebellar structures. Often, Chiari malformations are accompanied by spinal cord cavities known as syringomyelia. In most cases, individuals with Chiari malformations have a small posterior fossa, causing neural elements to be compressed and crowded at the foramen magnum.
Classification: Chiari malformations were initially described by John Cleland in 1883 and later classified by Hans Chiari in 1891 into four distinct groups.
- Chiari Type I malformation (CM-I) is characterized by the abnormal shape and displacement of cerebellar tonsils below the level of the foramen magnum.
- Chiari Type II malformation (CM-II), also referred to as Arnold-Chiari malformation, involves the downward displacement of the cerebellar vermis and tonsils, brainstem malformation with a beaked midbrain in neuroimaging, and spinal myelomeningocele.
- Chiari Type III malformation (CM-III) is exceedingly rare and combines a small posterior fossa with a high cervical or occipital encephalocele, often involving the displacement of cerebellar structures into the encephalocele and inferior displacement of the brainstem into the spinal canal.
- Chiari Type IV malformation (CM-IV) is now considered an outdated term describing cerebellar hypoplasia unrelated to the other Chiari malformations.
Additional subtypes, although less widely recognized, have since been defined, such as Chiari 0 malformation, characterized by brainstem anomalies (posterior pontine tilt, medulla downward displacement, low-lying obex) but normally positioned cerebellar tonsils, and Chiari 1.5 malformation, which resembles CM-II but lacks spina bifida. Both subtypes exhibit crowding at the foramen magnum.
Chiari I Anatomy:
Chiari malformation-I is characterized by abnormally shaped cerebellar tonsils displaced below the foramen magnum. In adults, it is considered normal for tonsils to extend up to 3 mm below the foramen magnum. However, tonsils lying 5 mm or more below this point on neuroimaging are typically indicative of Chiari malformation. The degree of tonsillar displacement does not necessarily correlate with clinical severity. In infants, tonsils as low as 6 mm below the foramen magnum can still be within the normal range.
The prevalence of syringomyelia in Chiari malformation-I cases varies, with one series reporting a 35 percent incidence in 415 symptomatic children. Some patients may have a holo-cord syrinx that spans the entire spinal cord length.
Atlas assimilation, commonly associated with the Klippel-Feil anomaly, is frequently observed in Chiari malformation-I. This anomaly may not be evident in infants and young children, as the fusion site may still be cartilaginous at an early age.
Chiari II Anatomy:
Chiari malformation-II is characterized by the downward displacement of the inferior cerebellar vermis, including the nodulus, pyramis, and uvula, as well as cerebellar tonsils and the medulla, through the foramen magnum into the upper cervical canal. This condition is typically associated with myelomeningocele at the lumbosacral or occasionally higher spinal cord levels, leading to hydrocephalus. Nearly all myelomeningocele patients have Chiari malformation-II, often accompanied by hydrocephalus. Constant features include a reduced posterior fossa volume, an enlarged foramen magnum, and a ventral displacement of the tentorium cerebelli.
Additional findings associated with Chiari malformation-II may include:
- Inadequate removal of the 4th ventricle into the upper cervical canal.
- Elongation and thinning of the lower pons and medulla.
- Beaking of the quadrigeminal plate.
- Kinking of the medullary spinal cord junction in the cervical canal.
- Stenosis or atresia of the cerebral aqueduct.
- Upward removal of the upper cerebellum into the intermediate fossa.
- Cerebellar dysplasia.
- Colpocephaly (irregular growth) of the posterior lateral ventricles.
Infrequent supratentorial anomalies that may accompany Chiari malformation-I and Chiari malformation-II include corpus callosum dysgenesis or absence, septum pellucidum agenesis, polymicrogyria of cerebral hemispheres, and cerebral gray matter heterotopia. Additionally, Chiari malformation-II is rarely linked to rhombencephalosynapsis, characterized by cerebellar vermis agenesis and fusion of cerebellar hemispheres.
Chiari III Anatomy:
Chiari malformation-III, an exceptionally rare condition, involves the downward displacement of the medulla into the upper cervical or occipital encephalocele. This encephalocele typically contains a significant portion of the cerebellum and may also include supratentorial tissue, such as the occipital cortex and a portion of the lateral ventricle’s occipital horn. Additionally, Chiari malformation-III may exhibit features present in Chiari malformation-I and Chiari malformation-II.
Causes and Pathophysiology of Chiari Malformation
The underlying causes and mechanisms behind congenital Chiari malformations continue to be a subject of ongoing debate. Several theories have been proposed, though none can comprehensively account for all aspects of this condition.
- Molecular Genetic Theory: This theory suggests that Chiari malformations result from inherent defects in the genetic processes governing the development of the hindbrain and the growth of related bone and cranial structures. Another theory linked to growth abnormalities posits that a clash between cranial growth directed caudally and cervical ripening directed rostrally underlies Chiari type I malformation (CM-I). However, it’s worth noting that most Chiari malformations are sporadic and not hereditary. Hence, the cause may involve a spontaneous genetic mutation or deletion or even a mutation triggered by external teratogenic factors.
- Crowding Theory: This theory proposes that a restricted growth of the posterior fossa leads to the compression of neural tissue, which is then pushed through the foramen magnum, akin to toothpaste squeezed through a tube. This theory is supported by observations of an abnormally small posterior fossa and downward displacement of the torcula in individuals with Chiari malformations.
- Hydrodynamic Pulsion Theory: According to this theory, progressive fetal hydrocephalus during early development exerts pressure on the brainstem and cerebellum, forcing them downward.
- Oligo-Cerebrospinal Fluid Theory: This theory posits that incomplete closure of the neural tube during early fetal development results in cerebrospinal fluid (CSF) leakage, leading to inadequate CSF volume to fully expand the embryonic ventricular system. This, in turn, results in a small posterior fossa and disorganization within the brain.
- Tethered Cord Theory: An earlier theory suggested that the downward displacement of cerebellar tissue was due to traction caused by a tethered spinal cord. However, subsequent studies have shown that traction is transmitted only up to the caudal-most pair of dentate ligaments, challenging the notion that it extends to the brainstem and cerebellum. Moreover, not all Chiari malformations are associated with tethering.
- Neuroectodermal and Mesodermal Origin: Chiari malformation-I can have either neuroectodermal or mesodermal origins. Isolated Chiari malformation-I is generally believed to have a mesodermal origin. In contrast, Chiari malformation-I associated with craniosynostosis syndromes, neurological disorders such as intellectual disability and epilepsy, is thought to stem from neuroectodermal factors. Occasionally, Chiari malformation-I can be linked to occult spinal dysraphism, specifically spinal bifida occulta. The remaining Chiari malformation types are primarily attributed to neuroectodermal anomalies.
The pathogenesis of spinal cord cavitations (syringomyelia) associated with Chiari malformations has also been a subject of debate. Initially, it was thought to result from the forced entry of CSF into the central canal due to impaired subarachnoid circulation at the foramen magnum level. However, studies using cine phase-contrast magnetic resonance imaging have revealed that the syrinx in Chiari malformations is non-communicating. Thus, it is more likely that the syrinx arises from craniospinal pressure dissociation due to the blockage of CSF flow within the subarachnoid space at the foramen magnum level. This leads to a backup of pressure into the venous system, initially engorging the Virchow-Robin spaces. Excess fluid then disperses into the spinal cord, resulting in spinal cord edema. As fluid accumulates beyond the resorption capacity of the parenchyma, it eventually fills the central canal, leading to the formation of a syrinx.
Epidemiology of Chiari Malformation
Prevalence: The precise prevalence of Chiari type I malformation (CM-I) remains somewhat elusive. In the era of magnetic resonance imaging (MRI), which began around 1985, there has been an increasing detection rate of Chiari malformation-I. Studies utilizing MRI have reported a prevalence ranging from 1 to 3.6 percent.
Traditionally thought to primarily affect adolescents and adults, it has become apparent that Chiari malformation-I can also manifest in younger children. Before the advent of MRI and its widespread use, diagnoses of Chiari malformation-I were typically made when patients displayed symptoms warranting further investigation. Consequently, early series often did not include children under 12 years of age, leading to the misconception that Chiari malformation-I primarily occurred in older age groups. However, with the broader application of MRI, individuals with asymptomatic or minimally symptomatic Chiari malformation-I are being identified at younger ages. Thus, Chiari malformation-I now stands as the most frequently diagnosed type of Chiari malformation in clinical practice. Chiari type II malformation, on the other hand, remains the most common type of Chiari malformation presenting in childhood.
Associated Conditions: Retrospective studies have linked Chiari malformations with certain medical conditions. For instance, Chiari malformation-I has been observed in association with Robin sequence, neurofibromatosis type 1, and Noonan syndrome. As an illustration, in a study encompassing 198 patients with neurofibromatosis type 1 who underwent neuroimaging, Chiari malformation-I was detected in approximately 9 percent of cases. Furthermore, among 130 patients who underwent surgical intervention for Chiari malformation-I, approximately 5 percent were diagnosed with neurofibromatosis type 1.
Clinical Manifestations of Chiari Malformation
Chiari I Clinical Features:
The true natural progression of Chiari type I malformation (CM-I) remains a matter of ongoing investigation. In many cases, Chiari malformation-I remains asymptomatic until adolescence or adulthood, often with a gradual onset. A study involving 43 patients with Chiari malformation-I found that the average age at presentation was approximately 18 years.
While Chiari malformation-I is typically asymptomatic in young children, retrospective analyses have identified 39 patients younger than six years who underwent early surgical treatment. Children aged up to two years often presented with oropharyngeal dysfunction, while those between three and five years more frequently exhibited syringomyelia, scoliosis, or headaches.
Pain or headaches are the most common presentations in older children and adults with Chiari malformation-I.
The clinical manifestations of Chiari malformation-I encompass the following categories:
- Asymptomatic: Some individuals are asymptomatic, with Chiari malformation-I incidentally discovered on magnetic resonance imaging (MRI), often during evaluations for various reasons, including seizures, nonspecific headaches, trauma, or developmental delay.
- Cerebellar Dysfunction: Symptoms can include nystagmus, scanning speech, and ataxia, with truncal ataxia being more prevalent than appendicular ataxia.
- Cranial Neuropathies/Brainstem Compression: These can manifest as hoarseness, vocal cord paralysis, dysarthria, palatal weakness, pharyngeal achalasia, tongue atrophy, recurrent aspiration, nystagmus (particularly down-beating), or sleep-related breathing disorders like central and obstructive sleep apnea. Less common symptoms include oscillopsia, sensorineural hearing loss, sinus bradycardia, syncope, and hiccups. Brainstem compression can also lead to long-tract signs such as weakness, spasticity, hyperreflexia, and Babinski responses, resulting from either brainstem or spinal cord compression.
- Headache: Pain or headache, attributed to meningeal irritation, is the most prevalent symptom in older children and adults with Chiari malformation-I. The pain is often occipital or nuchal and tends to be paroxysmal, though it can also be dull and persistent. Activities that increase intracranial pressure, such as coughing, laughing, or sneezing, exacerbate both pain and dizziness, leading to cough headaches. According to the International Classification of Headache Disorders, 3rd edition (ICHD-3), Chiari malformation-I-related headaches are triggered by cough or other Valsalva maneuvers, localized to the occipital or suboccipital region, and typically last under five minutes.
- Hydrocephalus: Some cases of Chiari malformation-I are associated with obstructive hydrocephalus, with a prevalence of approximately 10 percent in one small study. Symptoms in children often include headaches, irritability, behavioral changes, developmental delays, vomiting, and lethargy.
- Myelopathy: Myelopathy can manifest as motor weakness, sensory disturbances, or autonomic dysfunction. This may result from the compression of the cervical spinal cord by herniated cerebellar tonsils or the effects of a syrinx.
- Oropharyngeal Dysfunction: Typically seen in children under three years old, this dysfunction may present as sleep apnea or feeding difficulties.
- Scoliosis: Scoliosis often results from an asymmetric spinal syrinx, leading to differential growth of the hemicords and vertebral column. Scoliosis is now frequently diagnosed before the classic cape-like sensory loss occurs.
- Sleep-Related Breathing Disorders: Emerging data suggest that both central and obstructive sleep apnea can be associated with Chiari malformation-I in both children and adults.
- Syringomyelia (Syrinx): Syringomyelia, often accompanied by scoliosis, occurs in around 35 percent of individuals with symptomatic Chiari malformation-I. Signs and symptoms include gait disturbances, radicular pain, dysesthesia, paroxysmal pruritus, upper motor neuron signs in the legs, and lower motor neuron signs predominantly in the arms, particularly if the syrinx is located in the cervical region, the most common site associated with Chiari malformation-I. Brainstem dysfunction signs may also be present if the syrinx extends into the medulla (syringobulbia).
Presyrinx is a potentially reversible condition characterized by spinal cord edema due to cerebrospinal fluid flow obstruction. Typically found in the cervical region, it appears similar to a true syrinx on T2-weighted MRI images but lacks discrete cavitation on T1-weighted sequences.
Chiari II Clinical Features:
Chiari type II malformation (CM-II) is almost always associated with lumbosacral or thoracic myelomeningocele, making it typically detectable prenatally or at birth.
Symptoms in infancy may include dysphagia, arm weakness, stridor, apneic episodes, and aspiration. In late infancy and childhood, progressive hydrocephalus is a common issue in Chiari malformation-II. Additionally, Chiari malformation-II may be accompanied by one or more syndromes linked to Chiari malformation-I, such as syringomyelia and scoliosis.
Despite the extensive malformations, some individuals with Chiari malformation-II have normal intelligence and achieve independent functioning.
Chiari III Clinical Features:
Chiari malformation-III is exceedingly rare and is characterized by a high cervical or occipital encephalocele, which typically contains a substantial portion of the cerebellum, along with supratentorial tissue, including the occipital cortex and a segment of the occipital horn of the lateral ventricle. Data on the natural history and clinical course of Chiari type III malformation (CM-III) are limited. Infants with Chiari malformation-III often face a high mortality rate, frequently due to respiratory failure. Those who survive beyond the neonatal stage often experience severe neurological impairments, including intellectual disability, epilepsy, hypotonia or spasticity, upper and lower motor neuron signs, and lower cranial nerve deficits.
Evaluation and Diagnosis of Chiari Malformation
The diagnosis of Chiari malformations primarily relies on neuroanatomy, as there are no specific biomarkers in blood, cerebrospinal fluid (CSF), or cultured tissue to confirm the condition. Consequently, neuroimaging plays a pivotal role in the diagnostic process.
Imaging:
- MRI of Brain and Spinal Cord: Magnetic resonance imaging (MRI) of both the brain and the entire spinal cord stands as the gold standard for evaluating Chiari malformations. Multiple views, including sagittal, coronal, and axial images of the brain, along with sagittal and axial scans of the complete spinal cord (cervical, thoracic, and lumbar), employing T1- and T2-weighted MRI sequences, are essential for detecting cerebellar and brainstem displacement, associated abnormalities at the craniocervical junction, and the presence of hydrosyringomyelia.
- CSF Flow Imaging: In cases of Chiari malformation-I, it is advisable to conduct phase contrast cine MRI to assess cerebrospinal fluid (CSF) flow dynamics across the foramen magnum. This information can help guide decisions regarding surgical decompression of the foramen magnum to restore normal CSF flow. The role of phase contrast cine MRI in assessing CSF flow dynamics and managing Chiari malformation-I is discussed further below.
- CT scan: In situations where MRI is not feasible, a high-resolution computed tomography (CT) scan, complete with sagittal reconstructions, can be employed to diagnose Chiari malformation. CT, especially with thin-section multiplanar scans and reformatted images, remains valuable in evaluating associated bony abnormalities.
- Fetal Ultrasound: In select cases of fetal ventriculomegaly, a Chiari malformation may be detected prenatally using fetal ultrasound.
Diagnosis of Chiari Malformation-I:
Experts widely agree that Chiari malformation-I can be radiologically diagnosed in adolescents and adults through MRI when one or both cerebellar tonsils are displaced by ≥5 mm below the foramen magnum. Borderline displacement of cerebellar tonsils (≥3 to <5 mm below the foramen magnum) is considered pathologic if additional Chiari malformation-I features, such as other craniocervical junction anomalies or syringomyelia, are present.
A study comparing 200 normal subjects and 25 patients with Chiari malformation-I found that using a cutoff of 3 mm below the foramen magnum as the lowest normal position for cerebellar tonsils predicted symptomatic Chiari malformation-I with 96 percent sensitivity and 99.5 percent specificity. In patients with Chiari malformation-I, the mean position of cerebellar tonsils was 13 mm below the foramen magnum (ranging from 3 to 29 mm below). Conversely, in normal subjects, the mean position of the tonsils was 1 mm above the foramen magnum (ranging from 8 mm above to 5 mm below). In infants, tonsils as low as 6 mm below the foramen magnum may still be within the normal range, as studies have shown that cerebellar tonsils tend to ascend with age.
Diagnosis of Chiari Malformation-II:
Chiari type II malformation (CM-II) should be suspected in a fetus or newborn displaying clinical evidence of spinal myelomeningocele. Confirmation of the diagnosis is achieved through MRI, which demonstrates the downward displacement of the inferior cerebellar vermis and medulla through the foramen magnum into the upper cervical canal.
Diagnosis of Chiari Malformation-III:
Similarly, the diagnosis of Chiari type III malformation (CM-III) is established in a fetus or newborn with clinical evidence of a high cervical/occipital encephalocele. Confirmatory MRI reveals the inferior displacement of the medulla and the presence of a high cervical or occipital encephalocele with descent of cerebellar structures into the malformation.
Neurologic Assessment and Follow-up:
Patients with Chiari malformation-I should undergo assessment and follow-up by a neurologist to ascertain the symptomatic status of Chiari malformation-I. This is especially critical for characterizing headaches, as erroneously attributing primary headaches (e.g., migraines) to Chiari malformation-I could lead to unwarranted surgical interventions.
It is worth noting that Chiari malformation-I has not been linked to epilepsy or autism.
Sleep Evaluation:
Polysomnography should be considered for children or adults with Chiari malformation-I who present with respiratory symptoms suggestive of sleep apnea or severe tonsillar herniation.
Conditions Associated with Chiari Malformation
Children diagnosed with Chiari malformation often experience associated conditions, underscoring the importance of comprehensive care from a multidisciplinary team of medical professionals. This team may include specialists in pediatric neurosurgery, neurology, otolaryngology, and other relevant fields of medicine.
Conditions Associated with Chiari I:
Patients with Chiari I may develop a syrinx, scoliosis, or even a tethered spinal cord.
- Syrinx: A syrinx refers to a fluid-filled cavity within the spinal cord, which can lead to various neurological issues such as muscle stiffness, weakness, and pain. Typically, surgeons do not perform operations specifically to correct the syrinx. Instead, surgery to address the Chiari malformation itself can often alleviate these symptoms.
- Scoliosis: Scoliosis involves an abnormal curvature of the spine. In some cases, treating the Chiari malformation can influence the progression of scoliosis. However, there are instances where children may require additional surgical interventions to address the scoliosis independently.
- Tethered Spinal Cord: In rare occurrences, a child with Chiari malformation may also have a tethered spinal cord. This condition signifies that the spinal cord abnormally adheres to the surrounding tissues near the spine. Correcting the tethered cord may necessitate separate surgical procedures in addition to Chiari malformation treatment.
Furthermore, it’s worth noting that children diagnosed with Ehlers-Danlos syndrome (EDS), a connective tissue disorder characterized by joint hypermobility and instability, maybe at an elevated risk of developing Chiari I malformation.
Conditions Associated with Chiari II:
Chiari II malformation is commonly linked to two serious conditions: myelomeningocele and hydrocephalus, both requiring a collaborative approach involving a multidisciplinary medical team.
- Myelomeningocele: Myelomeningocele represents a severe form of spina bifida, where a portion of the spinal cord and surrounding nerves protrude through an opening in the spine. Children with myelomeningocele often experience additional medical challenges, including hydrocephalus and heart defects.
- Hydrocephalus: Hydrocephalus results from an accumulation of cerebrospinal fluid within the brain and spinal cord.
In addition to these conditions, some children diagnosed with either Chiari I or II may also experience various forms of sleep apnea, a disorder characterized by temporary breathing cessation during sleep. Addressing the Chiari malformation can sometimes alleviate or reduce sleep apnea symptoms.
Management of Chiari Malformation
Chiari Type I Malformation:
The management of Chiari malformations depends on the nature of the malformation and the degree of associated neurological impairments.
Asymptomatic Patients
On magnetic resonance imaging (MRI) of the brain or cervical spine performed for another reason, a Chiari Type I malformation (CM-I) may be discovered by accident.
- Asymptomatic without Syringomyelia: Patients with an incidental diagnosis of Chiari malformation Type I who are asymptomatic and who do not exhibit syringomyelia or obstruction of the cerebrospinal fluid (CSF) flow on phase-contrast MRI can be handled conservatively with clinical and MRI surveillance as necessary. In our practise, we only repeat the MRI for these patients if the clinical findings point to the potential emergence of Chiari malformation Type I symptoms or signs.
- Asymptomatic with Syringomyelia: For asymptomatic or mildly symptomatic patients with Chiari malformation Type I who are neurologically intact but have syringomyelia on MRI, management is controversial. There are sporadic examples of tonsillar displacement or syringomyelia spontaneously resolving in the context of Chiari malformation Type I, as is mentioned below. As a result, some have claimed that a time of careful observation is necessary for youngsters who are asymptomatic.
In one report of 218 children with incidentally discovered Chiari malformation-I, the discovery of occult neurological dysfunction on evaluation or identification of a syrinx on MRI led to early (within six months of presentation) decompressive surgery for 22 patients (10 %); subsequent development of de novo symptoms or syrinx led to later (after 6 months) surgery for another 14 patients (6 %).
CSF Flow Obstruction
We suggest obtaining a phase contrast cine MRI for patients with Chiari malformation Type I to look for CSF flow obstruction.
We refer patients for surgery if the phase contrast cine MRI reveals total CSF flow blockage.
We repeat the examination six months later if the initial analysis reveals a syrinx and a partial CSF flow restriction. If the clinical and imaging results are stable, we repeat the imaging one year later, then once more one to two years later, and as necessary after that.
We repeat imaging at one year and as needed after if stable at one year if the study only reveals partial blockage and no syrinx.
At the first sign of clinical worsening, we would refer patients for a neurosurgical examination and posterior fossa decompression.
In limited uncontrolled investigations, CSF flow obstruction was linked to clinical complaints; also, an increase in CSF flow following surgery was linked to clinical improvement or stabilization. Phase contrast cine MRI may also be useful for postoperative follow-up of patients with “failed” surgeries, such as those who experience delayed deterioration. Furthermore, a retrospective report of 130 patients who had decompressive surgery for Chiari malformation Type I found that normal CSF flow by phase contrast cine MRI before surgery was predictive of symptom recurrence after surgery.
Symptomatic Patients
Decompressive surgery is indicated for patients with Chiari malformation-I who are symptomatic with lower cranial nerve palsies, syringomyelia, myelopathy, cerebellar symptoms, or occipital cough headache.
- Role of Surgery:
- As noted above, we refer patients with Chiari malformation Type I for neurosurgical evaluation if they are symptomatic or have imaging evidence of a large syrinx or complete CSF flow obstruction.
- Decompression of the craniocervical junction and restoration of normal CSF flow in the vicinity of the foramen magnum are the two objectives of surgery for Chiari malformations. The most frequent operation is suboccipital craniectomy with or without duraplasty for posterior decompression. Shunting and odontoidectomy for anterior foramen magnum decompression are other techniques.
- Posterior Foramen Magnum Decompression:
- Decompression with a dural incision and decompression without a dural opening are the two primary surgical methods for posterior fossa decompression. For the best decompression, most surgeons conduct posterior fossa decompression with surgical opening of the dural sac. A limited suboccipital craniectomy, a C1 laminectomy, a duraplasty, and an arachnoid dissection are performed during the procedure. All 31 multidisciplinary specialists supported opening the dural sac for patients with Chiari malformation Type I and syringomyelia in a 2021 international consensus document on the diagnosis and treatment of the condition, while 75% supported this course of action for patients with symptomatic Chiari malformation Type I without syringomyelia. Meningitis, the development of pseudomeningoceles, CSF leaking, acute postoperative hydrocephalus, and others are possible side effects.
- Decompression of the bony posterior fossa without opening the dural sac is a more conservative method. Avoiding CSF-related problems such as CSF leak, pseudomeningocele, and aseptic meningitis is the method’s main potential benefit.
- The information that is currently available, despite being largely retrospective and uncontrolled, suggests that posterior fossa decompression with duraplasty is linked to a higher rate of clinical improvement, especially for patients with syringomyelia, and a lower rate of the need for reoperation when compared to the more conservative surgery without duraplasty, but an increased rate of CSF-related complications. A lower rate of CSF-related problems and a higher rate of reoperation are linked to less invasive surgery without duraplasty.
- Due to the lack of standardization in the surgical methods used by the included studies and the lack of blinding in the outcome evaluation, it is unclear if the studies comparing decompression with and without duraplasty are generalizable. The complication rates for various hospitals and surgeons also vary greatly. For instance, a retrospective cohort of 40 patients with Chiari malformation Type I who had decompression with duraplasty found a low perioperative complication rate (3%), which was related to the development of a pseudomeningocele in one patient. The patients’ ages ranged from 3 to 45 years, with the mean being 13.3 years. Meningitis, CSF leak, or postoperative hydrocephalus episodes were nonexistent.
- Anterior Foramen Magnum Decompression:
- An alternate surgical method for treating Chiari malformations is anterior decompression of the foramen magnum, commonly accomplished with transoral odontoidectomy. Patients with craniovertebral junction abnormalities (such as basilar invagination) who do not respond to posterior decompression are those who most frequently use it. For individuals who have severe ventral brainstem compression due to a Chiari malformation, anterior decompression has also been utilized alone or in conjunction with posterior decompression.
- Shunting Procedures:
- Syrinx shunt placement has been used mainly for patients with Chiari malformation Type I who fail posterior decompression due to progressive symptoms or syrinx enlargement.
Management of Chiari Type II and III Malformation:
Referrals for neurosurgical examination should be made for patients with Chiari Type II malformation (CM-II) and Chiari Type III malformation (CM-III). Surgical therapies may include closing open neural tube defects soon after birth, treating hydrocephalus (often with a shunt), and decompressing tight posterior fossa structures, in particular for Chiari malformations-II and Chiari malformation-III. The care of neurogenic bowel and bladder, infant feeding challenges, respiratory failure, and apnea are medical concerns.
Prognosis of Chiari Malformation
The clinical course of Chiari Type I malformation (CM-I) can be quite unpredictable. While some patients may remain asymptomatic, others may experience spontaneous resolution of tonsillar displacement or spinal cord syringomyelia. On the flip side, some individuals may face a relentless worsening of their syrinx condition. Furthermore, long-standing disease may result in scarring, limiting the potential benefits of surgery, especially if the syrinx has been present for more than three years.
It’s worth noting that the natural history of Chiari malformation-I remains somewhat uncertain. Limited evidence suggests that most patients with minimal or no symptoms tend to remain stable. For instance, in one report, a group of 22 children with Chiari malformation-I who displayed mild or absent clinical manifestations were monitored without surgery for an average period of 5.9 years (ranging from 3 to 19 years). At their last follow-up, 17 patients (77 %) showed no symptoms or improvement, while five patients (23 %) experienced clinical deterioration. Among those, the deterioration was mild in two cases, while the remaining three required surgical intervention. Interestingly, only one child had syringomyelia at the start of the study, and this condition remained unchanged over 19 years of follow-up. However, cervical syringomyelia developed de novo in three children during the follow-up period.
The outcomes of surgical interventions for Chiari malformation-I can vary significantly. While there isn’t data from randomized controlled trials available, retrospective series suggest that the majority of patients who undergo posterior fossa decompression, with or without duraplasty, tend to experience postoperative improvement or stabilization.
For instance, in one retrospective series involving 157 patients (with a mean age of 38 years, ranging from 16 to 75 years) who had Chiari-related syringomyelia and underwent posterior fossa decompression with dural opening, the results were as follows: at a median follow-up of 88 months, clinical improvement or stabilization was observed in 63 percent and 31 percent, respectively, while deterioration or death occurred in 6 percent and 1 percent, respectively. Factors associated with a poor outcome included older age at the time of surgery and the presence of a long-tract deficit. Surprisingly, the extent of the syrinx and the degree of tonsillar descent before surgery were not correlated with the outcomes. However, postoperative magnetic resonance imaging did reveal that syrinx size was a predictor of poorer outcomes.
Summary and Recommendations
Anatomy and Classification:
There are 3 major types of Chiari malformations:
- Chiari I Malformation (CM-I) is characterized by the abnormal displacement of cerebellar tonsils below the foramen magnum.
- Chiari II Malformation (CM-II) involves the downward displacement of the cerebellar vermis and tonsils, a brainstem malformation with a beaked midbrain on neuroimaging, and a spinal myelomeningocele.
- Chiari III Malformation (CM-III) is rare and combines a small posterior fossa with a high cervical or occipital encephalocele, often involving displacement of cerebellar structures into the encephalocele and inferior expulsion of the brainstem into the spinal canal of the spine.
Epidemiology:
Chiari malformation-I is the most common among the Chiari malformations. The prevalence of Chiari malformation-I remains unknown but may be around 1% or higher.
Clinical Manifestations:
Chiari malformation-I is frequently asymptomatic, although it can manifest with various symptoms, including cough, headache, syringomyelia, cerebellar dysfunction, brainstem-related symptoms, oropharyngeal dysfunction, cranial neuropathies, hydrocephalus, myelopathy, or scoliosis. In infants, Chiari malformation-II may present with dysphagia, arm weakness, stridor, apneic spells, aspiration, and later development of hydrocephalus. Chiari malformation-III is associated with high mortality in infancy due to respiratory failure and severe neurologic impairments in survivors.
Evaluation and Diagnosis:
Magnetic resonance imaging (MRI) is the preferred imaging modality for diagnosing Chiari malformations:
- In adolescents and adults, Chiari malformation-I is diagnosed via MRI when one or both cerebellar tonsils are displaced ≥5 mm below the foramen magnum.
- Chiari malformation-II is diagnosed in fetuses or newborns with spinal myelomeningocele and MRI findings of inferior cerebellar vermis and medulla displacement through the foramen magnum.
- Chiari malformation-III is identified in fetuses or newborns with high cervical/occipital encephalocele and MRI showing the descent of cerebellar structures into the malformation.
Management:
Management strategies for Chiari malformations depend on the specific malformation and the extent of associated neurological impairments:
- Asymptomatic patients incidentally diagnosed with Chiari malformation-I without syringomyelia or cerebrospinal fluid (CSF) flow obstruction can undergo conservative management with clinical and MRI surveillance.
- Asymptomatic or mildly symptomatic Chiari malformation-I patients should undergo MRI of the entire spine with a phase contrast cine MRI. Surgical referral is considered if significant syrinx or complete CSF flow obstruction is identified. Otherwise, clinical and neuroimaging surveillance is recommended.
- Symptomatic Chiari malformation-I patients with lower cranial nerve palsies, myelopathy, cerebellar symptoms, or occipital headaches related to the malformation or associated syringomyelia should be referred for surgical evaluation, including foramen magnum decompression.
- For Chiari malformation-II and Chiari malformation-III, surgical interventions may involve the closure of open neural tube defects shortly after birth, treatment for hydrocephalus, and decompression of tight posterior fossa structures. Medical management is necessary for issues such as neurogenic bowel and bladder, neonatal feeding difficulties, respiratory failure, and apnea.
Outcomes:
The clinical course of Chiari malformation-I is uncertain and can vary widely. Limited evidence suggests that most patients with minimal or no symptoms tend to remain stable. In retrospective series, the majority of patients who undergo posterior fossa decompression report postoperative improvement or stabilization.
FAQ
Is a Chiari malformation serious?
The symptoms of type II Chiari malformation, which is typically more severe than type I, typically appear in childhood. Chiari malformation type II can range widely in severity. During infancy or childhood, the disease may result in serious, potentially fatal complications.
What Is the Life Expectancy for Chiari Malformation?
The type of Chiari malformation will determine how long it will last. Patients with Chiari type I malformation, the mildest form of the illness, are frequently diagnosed as adults, have a normal life expectancy, and have positive surgical and/or therapeutic outcomes.
How is Chiari diagnosed?
Due to the ambiguity or absence of symptoms, Chiari malformations can be challenging to identify. An MRI scan, which shows the cerebellum’s aberrant extension towards the spinal cord, is typically required to provide a conclusive diagnosis.
Is Chiari curable?
Surgery to correct the defect and treat symptoms is the mainstay of Chiari I treatment for the majority of patients. Patients who do not have symptoms, however, might not require surgery. Instead, they visit their doctors often for follow-up exams.
Is Chiari a brain disease?
Chiari malformations (CM) are structural flaws where the cerebellum and base of the skull meet and the lower half of the brain presses through the opening into the spinal canal. The area of the brain that regulates balance is called the cerebellum.
How do you prevent Chiari?
It cannot be stopped. Spina bifida and Chiari malformation can co-occur occasionally. By taking folic acid, women who are pregnant or intend to become pregnant may be able to reduce their child’s risk of developing spina bifida. Before taking folic acid or other supplements, see your doctor.
What is the risk factor of Chiari malformation?
Primary CM can result from a variety of sources, including genetic abnormalities that can lead to improper fetal development. Folic acid, among other essential vitamins and nutrients, may be lacking during pregnancy and have an impact on fetal development. Fetal development could be impacted by an infection or a high fever when pregnant.
Does Chiari affect memory?
One potential side effect of Chiari malformation or the procedure to treat it is cognitive damage. Diffuse cognitive deficiencies, such as issues with attention, memory, executive functioning, and information processing, may result from the condition and the operation, which may physically alter brain tissue.
When do Chiari’s symptoms start?
Signs and symptoms of Chiari malformation type 1 typically first show in late childhood or early adulthood. The most common and common severe symptom of Chiari malformation is headaches. They typically happen after a quick strain, cough, or sneeze.
What is the new treatment for Chiari malformation?
In order to increase the space surrounding the cerebellum and cervical spine during the decompression surgery, bone was removed. This helped to restore the normal flow of spinal fluid and reduce pressure on the spinal cord, stopping future injury. The patient no longer has headaches after the operation.
Reference
- Chiari malformation – Symptoms and causes – Mayo Clinic. (2021, September 24). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/chiari-malformation/symptoms-causes/syc-20354010#:~:text=Chiari%20malformation%20(kee%2DAH%2D,brain%20and%20forcing%20it%20downward.
- Chiari Malformation | Types, Symptoms, Diagnosis & Treatment. (n.d.). https://www.cincinnatichildrens.org/health/c/chiari-malformation
- UpToDate. (n.d.). UpToDate. https://www.uptodate.com/contents/chiari-malformations