Enteric Nervous System (ENS)
Introduction
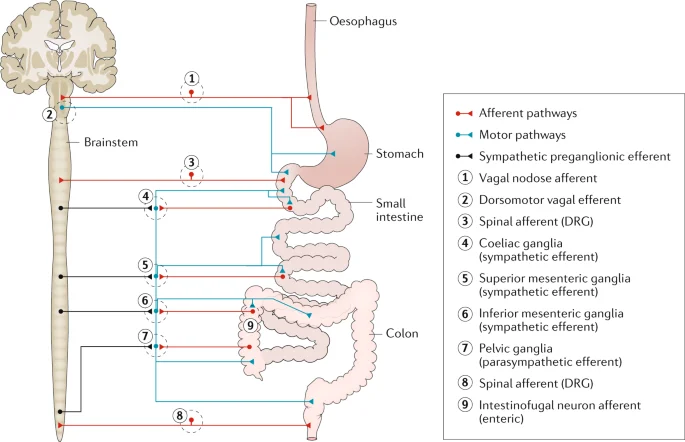
The nervous system of the stomach.
- Through connections with the sympathetic nervous system and central nervous system, the enteric nervous system (ENS) regulates the digestive system. The gastrointestinal system’s wall is covered in a network of sensory, motor, and interneurons that extends from the lower part of the esophagus all the way to the rectum. The submucosal and myenteric plexuses of the gut wall are where the neurons of the ENS are organized into two layers.
- Some estimations suggest that the Enteric Nervous System has more neurons than the total spinal cord.
- The autonomic nervous system’s input is combined with the ENS’s processing of a variety of feelings, including the type of gut contents and gut distension. The ENS can control and optimize the muscular and secretory functions of the gastrointestinal tract in this way.
- Many of the ENS effector neurons also get parasympathetic motor neuron innervation, making them parasympathetic effector neurons. Because of this, the ENS is recognized as a crucial component of the parasympathetic nervous system, however, it differs from a simple parasympathetic ganglion in that it has specialized sensory neurons and independent processing. The ENS exhibits complex coordination, flexibility, and learning in response to dietary changes or perturbations to the gut.
Types of Enteric Neurons
- According to their roles, enteric neurons can be divided into about 20 different categories (Brookes and Costa 2002; Furness 2006). Each type is defined by combinations of qualities, including morphology, neurochemical characteristics, cell physiology, and projections to targets. There are 20 different types of neurons, but only three groups can be distinguished: motor neurons, interneurons, and intrinsic primary afferent neurons (IPANs, also known as intrinsic sensory neurons). IPANs identify the chemical characteristics of the luminal contents and the physical state of the organs (e.g., the tension in the gut wall).
- They respond to these signals by starting the proper reflex control of blood flow, secretion, and motility. Interneurons, interneurons, and motor neurons are all connected by IPANs. Interneurons communicate with each other and with motor neurons. Muscle motor neurons, secretomotor neurons, vasodilator neurons, and secretomotor/vasodilator neurons are all types of motor neurons.
Gastrointestinal neurons
Preceptive afferent neurons
- Both the myenteric and submucous ganglia include primary afferent neurons, commonly known as enteric primary afferent neurons (EPANs) or intrinsic primary afferent neurons (IPANs). They react to radial stretch, muscular tension, mucosal mechanical deformation, and luminal chemical stimuli. It is not yet known whether serotonin or other chemicals are released by epithelial cells like enterochromaffin cells in response to chemical or mechanical stimuli to activate the terminals of primary afferent neurons. They make up 14% of submucosal neurons and roughly 30% of myenteric neurons have a distinctive Dogiel type II form and have action potentials with prolonged post-hyperpolarization periods.
- All of these neurons locally branch within the submucous and myenteric ganglia and project to the villi. Long descending connections to the aboral myenteric ganglia are also present in a fraction of these neurons (ten percent of primary afferent neurons).8 To create reciprocally innervated networks, they receive slow synaptic input from other primary afferent neurons, which is probably mediated by tachykinins. They circumferentially project to form synapses with the myenteric ascending, descending, longitudinal, excitatory, inhibitory, and circular muscle motor neurons. Numerous ascending and descending paths are probably used to connect certain subpopulations individually.
Circular muscle motorneurons excitatory
- These neurons represent the ultimate motor output to the circular muscle (14%), have a Dogiel type I shape, and receive fast nicotinic and possibly slow synaptic input from local primary afferent neurons as well as the only class of cholinergic ascending interneurons. They also appear to be stimulated by descending interneurons. They project to the circular muscle, where they join together to produce a denser network of nerve endings in the deep muscular plexus. They utilize acetylcholine and tachykinins as transmitters, acting directly on smooth muscle and maybe indirectly via the deep muscular plexus network of interstitial cells.
Circular muscle motorneurones inhibitory
- Fast nicotinic inputs from primary afferent neurons and non-cholinergic inputs from long descending primary afferent neurons are received by these Dogiel type I neurons (17%). They project to the circular muscle, where their axons connect with excitatory motorneurones in the deep muscular plexus. They use a variety of inhibitory transmission methods, including nitric oxide, adenosine triphosphate, and the peptides vasoactive intestinal peptide (VIP) and pituitary activating cyclic AMP peptide, which operates directly on smooth muscle or indirectly through interstitial cells.
Motorneurons of longitudinal muscle
- This relatively substantial class (25%) of tiny neurons with short projections to longitudinal muscle receive synaptic inputs from enteric primary afferent neurons as well as ascending and descending pathways.
Rising interneurons
- This tiny (5%) but a crucial class of enteric neurons has a Dogiel type I morphology and gets fast synaptic inputs from other ascending interneurons in a chain of ascending excitation. They also receive nicotinic synaptic inputs from enteric primary afferent neurons, both fast and slow. They communicate with the last excitatory circular muscle motor neurons via fast nicotinic and non-cholinergic slow synaptic inputs as they project orally within the myenteric plexus. They contain not only the enzyme for acetylcholine synthesis but also tachykinins and opioid peptides.
Motorneurons of longitudinal muscles
- This relatively substantial class (25%) of tiny neurons with short projections to longitudinal muscle receive synaptic inputs from enteric primary afferent neurons as well as ascending and descending pathways.
Descending interneurons
- DESCENDING INTERNEURONES
- There are various kinds of descending interneurons, which account for around 7% of all interneurones.6 14 Three of these are most likely cholinergic since they contain the acetylcholine production enzyme, choline acetyltransferase (ChAT). Each has a unique neurochemistry. Descending interneurons (4%) containing somatostatin and ChAT have a filamentous shape, receive fast and slow synaptic inputs primarily from non-primary afferent neurons, and form a chain of interconnected interneurons synapsing with other somatostatin neurons as well as myenteric and submucous neurons. Neurons expressing serotonin and ChAT (2%) project aborally to other myenteric and submucosal neurons but not to inhibitory motorneurones. It is unknown whether these neurons utilize serotonin in addition to acetylcholine. Serotonin can function either through quick ion channel gated receptors or through slow G protein-linked receptors. Neurons carrying NOS, VIP, and ChAT also project aborally to synapse with neighboring myenteric neurons. Neurons that express NOS and VIP but lack ChAT also project additional aboral myenteric and possibly submucous ganglia. It is unknown whether these non-cholinergic interneurons utilize additional rapid synaptic transmitters such as adenosine triphosphate or glutamate.
- A possible functional connection between the motor, secretory, and vasomotor pathways can be seen in some of these interneurons’ dual projections to both the myenteric and submucous ganglia.
Neurons of the secretomotor and vasomotor
- In the myenteric ganglia, there are two tiny classes of secretomotor neurons (1% each). One is cholinergic, while the other is non-cholinergic and contains VIP. They radiate to the mucosa. Neurons with identical functions and neurochemistry can also be found in the submucous ganglia, where they account for 32% and 42% of the total. Some VIP submucous neurons project to the myenteric ganglia and may provide the foundation for a functional link between secretion and motility. Extrinsic sympathetic neurons and unidentified myenteric neurons send inhibitory synaptic inputs to the VIP secretomotor neurons. The majority of submucous neurons receive both fast and slow synaptic inputs. Submucous cholinergic neurons (12%) are tiny submucous neuron class that projects to the mucosa and local blood vessels.
Intestinofugal neurons
- Cholinergic neurons receive rapid synaptic inputs and project from the myenteric ganglia to the prevertebral ganglia.
Additional gastrointestinal regions
- The unusual polarity of enteric motor neurons and interneurons discovered in the small intestine are also found in the esophagus, stomach, and large intestine, indicating that it is a prominent and conserved aspect of the layout of the enteric neural pathways. There are also significant variances in the classes represented and their neurochemical coding between regions. In the stomach, for example, there are extremely few enteric primary afferent neurons of Dogiel type II.
Enteric neural networks
- The first advances in elucidating enteric neuronal circuits were made by adopting the standard approach of specific and localized stimulation and recording of reflex responses. The contemporary study of intestinal reflex pathways began with Bayliss and Starling’s (1899)15 demonstration of polarised responses to mechanical stimuli. They proposed the existence of short ascending excitatory routes and longer descending inhibitory channels, resulting in the concept of “the law of the intestine.” Analysis of such routes has evolved greatly in recent years (fig2). As a result, there are ascending excitatory routes including EPANs, a chain of short ascending interneurons, and the final excitatory motorneurones to the circular muscle. The descending inhibitory routes are most likely made up of a different type of EPAN, with lengthy anal projections connecting to the ultimate inhibitory motorneurone. Mechanical activation of EPANs, which synapse with local inhibitory and excitatory motorneurones, also activates circumferential networks. A descending excitatory pathway to the circular muscle also exists, which involves mechanically sensitive EPANs and final excitatory motorneurones. It is unknown whether these routes contain descending interneurons. Reflex circuits involving motor responses of the longitudinal muscle have received little attention. There are other reflex routes including the uncommon intestinofugal neurons, which are located in the myenteric ganglia and synapse with postganglionic sympathetic neurons projecting to the submucous and myenteric ganglia. They are brief intestine-intestinal inhibitory reflex circuits that reduce motor and secretomotor activity when activated.
- Secretomotor and vasomotor reactions are both triggered by similar mechanical and chemical inputs. EPANs in the submucous and myenteric ganglia, as well as cholinergic and non-cholinergic secretomotor and vasomotor neurons, are involved in the secretomotor and vasomotor neuronal pathways that underpin these reactions.
Enteric nervous system modular organization
- All kinds of enteric neurons are evenly distributed across the ganglia network. As a result, all of the intestinal pathways mentioned above form continuous overlapping networks. Small circular muscle rings can contract separately, which makes them and the corresponding enteric neurons into functional modules. The development of the varied repertoire of motor patterns is determined by the spatiotemporal coordination of these related modules.
Pattern of motorization
- The role of these reflex circuits in the formation of various motor patterns is an interesting question. Other motor patterns can be identified based on their reliance on intestinal contents, in addition to myogenic mechanisms that can cause rhythmic contractions and, in certain circumstances, propulsion.
Accommodation
- Local accommodation is widely recognized to occur in the stomach and large intestine. Furthermore, during filling, distension activates local pathways in the small intestine, most likely a combination of circumferential and descending inhibitory reflex pathways, which relax the circular muscle.
Persistent neural peristalis
- Peristalsis is the propulsion of contents caused by the consecutive contraction of the circular muscle begun by the intestinal material. When the intraluminal volume hits a particular threshold or certain substances are present in the lumen, this pattern appears. This propulsive behavioral pattern consists of a ring of circular muscular contractions that begin orally and progress aborally, pushing the contents forward.17 This stereotyped motor pattern involves the activation of ascending excitatory pathways, descending inhibitory pathways, and perhaps activation of descending excitatory pathways.18 19 It has been proposed, but not proved, that activation of the collaterals of the extrinsic primary spinal afferent neurons is required for peristalsis in the colon. The likely significance of such spinal sensory neuron collaterals in other motor patterns in normal and illness remains unknown. Further research is needed to determine the precise timing of each of these pathways’ activation. However, the commencement of peristalsis appears to require the explosive recruitment of enteric excitatory motorneurones, which is suggestive of self-reinforcing neuronal processes. Primary afferent neurons and ascending interneurons are likely among the neurons and mechanisms responsible for this explosive recruitment. Thus, rather than a simple reflex, peristalsis can be seen as a motor pattern, a type of intestinal locomotion based on neuromechanical feedback.
Motor complex in movement
- The neurally mediated migrating motor complex is one of the most fascinating and complicated neuronal activities outside of the central nervous system. In addition to the motor patterns dependent on intestinal material triggering the enteric reflex pathways (as discussed above), there are neuronal circuits within the ENS that activate circular muscle motor activity over large parts of the gut on their own.22 Final excitatory motorneurones are stimulated by other enteric neurons via nicotinic and non-nicotinic processes in the circuits involved in the production of the migrating motor complex. As this neuronal activity slowly migrates aborally, it is likely that some of the descending interneurons are responsible. Although this cyclic motor activity has only been observed in conscious animals in the small intestine, evidence suggests that more localized migratory motor activity occurs in isolated small and large intestine preparations. The segmenting motor pattern observed by previous researchers could be owing to more localized, slowly migrating brain activity and its interplay with myogenic rhythmic activity.
Functions of the enteric nervous system
Motility Control
- The exterior muscle coat of the gastrointestinal tract serves to move the digestive tube’s contents as well as mix the food so that it is exposed to the enzymes that break it down and the absorbent lining of the intestine. In order to accommodate the increasing bulk of the contents, especially in the stomach, the muscle also relaxes. The colon, in particular in humans, serves as a vital reservoir to hold onto feces till discharge. By regulating the activity of both excitatory and inhibitory neurons that innervate the muscle, the enteric reflex circuits regulate movement. For the excitatory neurons, acetylcholine and tachykinins, and for the inhibitory neurons, nitric oxide, vasoactive intestinal peptide (VIP), and ATP, there are co-transmitters in these neurons. Additionally, Additionally, there is proof that carbon monoxide (CO) and pituitary adenylate cyclase-activating peptide (PACAP) play a role in the transmission of inhibitory signals.
- Depending on the type of food consumed, including its quantity and nutrient content, the length of time it takes for the contents to move through the digestive system varies. Food travels from the mouth to the stomach in roughly 10 seconds due to the peristaltic movement of the esophagus, where it is combined with digestive fluids. After eating, the stomach empties over the course of about one to two hours, with the liquefied contents being pushed into the jejunum by tiny aspirations caused by gastric peristaltic waves. The liquid substance of the small intestine, known as chyme, is created by combining the fluid from the stomach with pancreatic and biliary secretions. Under the control of mixing and propulsive movements organized by the ENS, chyme is mixed and travels gently along the intestine. while nutritional digestion and absorption take place. The human small intestine takes 3–4 hours to complete one transit. In healthy humans, colonic transit takes 1-2 days.
- The production of the small and large intestine’s motility patterns depends on the enteric nervous system’s intrinsic reflexes. Mixing activity, short-distance propulsive reflexes, the migrating myoelectric complex, peristaltic rushes, and retropulsion related to vomiting are the main muscular actions in the small intestine. These various results are programmed into the enteric nervous system. Peristalsis in the stomach, in contrast to the intestine, results from conducted electrical events (slow waves) that are produced in the muscle. The vagus nerves’ actions, which result in connections with enteric neurons in the myenteric ganglia, control how strongly the stomach contracts. To prepare for the arrival of meals, the proximal stomach relaxes. Additionally, the connections between enteric neurons and the vagus nerve facilitate this relaxation. As a result, although the enteric nervous system contains the key integrative centers for controlling small and large intestines, the brainstem contains those for controlling gastric motility. In most mammals, including humans, the proximal half or more of the exterior wall of the esophagus’ contractile tissue is made of striated muscle. An integrative circuitry in the brainstem regulates the striated muscle portion of the esophagus via the vagus. The myenteric ganglia are therefore modifiers rather than crucial control centers for esophageal peristalsis, despite their prominence in the striated muscle portion of the esophagus.
- The transit of the luminal contents between areas is constrained and regulated by the smooth muscle sphincters. Reflexes that are activated distally prevent retrograde transit of contents into more proximal portions of the digestive system, whereas reflexes that are initiated proximally to the sphincters relax the sphincter muscle and enable the passage of the contents.
- When sympathetic nerve activity increases, the movement of the contents from the oral to the anal direction is inhibited. This is accomplished by inhibiting enteric excitatory reflex transmission to the muscle and contracting the sphincters. The main transmitter used by post-ganglionic sympathetic neurons is noradrenaline. The sympathetic pathways have little effect on motility when the body is at rest. When defensive reflexes are triggered, they go into action.
Control of blood flow and fluid exchange locally
- The transport of water and electrolytes between the gut lumen and tissue fluid compartments is regulated by the enteric nervous system. It accomplishes this by controlling the activity of secretomotor neurons, which govern the permeability of the small and The mucosa of the large intestine is sensitive to ions. Secretomotor neurons use acetylcholine and vasoactive intestinal peptide (VIP) as neurotransmitters. Vasodilatation, which contributes some of the fluid released, is integrated with secretion. Submucosal ganglia house the cell bodies of the majority of secretomotor neurons.
- Every day, fluid fluxes larger than the body’s total blood volume pass over the epithelial surfaces of the digestive tract. For the body’s fluid and electrolyte balance to be maintained, enteric nervous system control of this fluid movement is crucial. The small intestinal epithelium experiences the biggest fluxes, but there is also significant fluid movement in the large intestine, stomach, pancreas, and gallbladder. Osmotically active molecules are transferred between bodily fluid compartments and the lumens of the digestive organs, causing water to travel in both directions. In the small and large intestine, gallbladder, and pancreas, the largest secretion occurs in conjunction with outward fluxes of Cl and HCO3, while the greatest absorption of water, 8 to 9 liters per day, occurs in conjunction with the inward flux of nutrient molecules and Na+ through the activation of nutrient co-transporters. Enteric reflexes regulate the secretion of fluid from each of these organs. The reflex circuits of the enteric nervous system are inherent to the small intestine and the majority of the colon. They pull water from the absorbed fluid and the circulation while balancing secretion with absorptive fluxes. Through central reflex centers, inhibitory sympathetic nerve pathways that react to variations in blood pressure and blood volume govern the activation of the secretomotor reflexes in a physiologically significant manner.
- Local blood flow to the mucosa is regulated by enteric vasodilator neurons in order to balance the nutritional needs of the mucosa and accommodate fluid exchange between the vasculature, interstitial fluid, and gut lumen. There are no vasoconstrictor neurons in the body. The CNS regulates overall blood flow to the gut via sympathetic vasoconstrictor neurons. To distribute cardiac output in accordance with the relative needs of all organs, sympathetic vasoconstrictor neurons work in tandem with the autonomic control of other arterial bedsThe sympathetic nervous system can divert blood flow away from the gastrointestinal tract when necessary, such as during digestion.
Regulation of gastric and pancreatic secretion
- Gastric acid secretion is controlled by both neurons and hormones. Cholinergic neurons with cell bodies in the stomach wall regulate neural activity. These receive excitatory signals from both enteric and vagus nerves.
- The release of pancreatic enzymes and HCl and pepsinogen in the stomach is largely dependent on vago-vagal reflexes. The final common channel is formed by enteric motor neurons, however, the involvement of intrinsic reflexes is small. To neutralize the contents of the duodenum, pancreatic secretion of bicarbonate is controlled by secretin, a hormone secreted from the duodenum, in collaboration with the activity of cholinergic and non-cholinergic enteric neurons. Nerves also govern secretion into the gallbladder and bicarbonate secretion in the distal stomach.
gastrointestinal endocrine cell regulation
- In the mucosa of the digestive tract, nerve fibres are found close to endocrine cells, some of which are managed by the nervous system. For instance, excitatory neurons that use gastrin-releasing peptide as the primary neurotransmitter innervate the gastrin cells in the antrum of the stomach.
- Hormones secreted by gastrointestinal endocrine cells, on the other hand, regulate the terminals of enteric neurons. Endocrine cells function similarly to taste cells in that they sample the luminal environment and release messenger chemicals into the mucosa tissue, which contains nerve endings. Because the mucosal epithelium separates the nerve ends from the lumen, this connection is required. Communication with serotonin (5-hydroxytryptamine, 5-HT)-containing endocrine cells, which trigger motility reflexes, is critical. Excessive serotonin release can produce nausea and vomiting, therefore 5-HT3 receptor antagonists are anti-nauseants.
Defense reactions
- Enteric neurons play a role in a variety of gut defense reactions. When infections are present in the gut, defense mechanisms include diarrhea to dilute and eliminate toxins, increased colonic propulsive activity, and vomiting.
- Fluid secretion is triggered by unpleasant stimuli, particularly the intraluminal presence of some viruses, bacteria, and bacterial toxins. This secretion is caused in significant part by the stimulation of enteric secretomotor reflexes. The physiological goal is clearly to cleanse the body of infections and their byproducts. However, if the pathogens outnumber the body’s ability to deal, fluid loss (diarrhea) might pose a major hazard to the organism.
Reflexes of the stomach and intestine
- Hormones (such as cholecystokinin, gastrin, and secretin) and neuronal circuits both carry signals between gut areas. Entero-enteric reflexes govern how one region interacts with others. When nutrients enter the small intestine, for example, the pancreas secretes digesting enzymes. A specific regulation system for the gastrointestinal tract is provided by a set of nerve circuits that convey signals from one section of the intestine to the sympathetic ganglia and back to the gut wall. The afferent limbs of these reflexes are formed by neurons with cell bodies in the enteric ganglia and terminals in the pre-vertebral sympathetic ganglia. IFANs are intestinofugal afferent neurons (Szurszewski et al., 2002).
Interactions between the ENS and the CNS
- The gastrointestinal system communicates with the CNS in both directions. Afferent neurons transmit information about the status of the digestive system. Some of this reaches consciousness, such as pain and discomfort from the gut, as well as conscious emotions of hunger and satiety, which are integrated perceptions derived from the gastrointestinal tract and other signals (for example, blood glucose).
- Other afferent signals, such as the nutritional load in the small intestine or stomach pH, do not generally reach awareness. In turn, the CNS sends signals to the gut, which are usually conveyed by the ENS.
- The sight and smell of food, for example, causes preliminary actions in the gastrointestinal system, such as salivation and gastric acid release. The cephalic phase of digestion is the word for this.
- Food stimulates the pharynx and upper esophagus, eliciting afferent signals that are integrated into the brainstem and then transmit efferent signals to enteric neurons in the stomach, causing acid secretion and increased gastric capacity in preparation for the food’s arrival.
- Defecation centres in the spinal cord receive signals from the colon and rectum, which are then transferred to the colon, rectum, and anal sphincter to cause defecation.
- Higher CNS areas exert inhibitory control on the defecation centers, and this inhibition can be lifted when the decision to defecate is made. The other major impacts come from sympathetic pathways.
Pathology
- There are numerous illnesses linked with the neurological regulation of digestion, many of which result from enteric nervous system anomalies (De Giorgio and Camilleri 2004; Spiller and Grundy 2004). Hirschprung’s illness is a neuropathology of the gut that causes agenesis of the enteric nervous system that extends proximally from the rectum for varying distances. If left untreated, it is lethal. Hypertrophic pyloric stenosis, esophageal atresia, gastroparesis, slow transit constipation, some forms of esophageal reflux, and Chagas’ disease are other enteric neuropathologies. Irritable bowel syndrome (IBS) is commonly mistaken for enteric neuropathy, despite the fact that IBS encompasses a wide range of disorders.
Relationships between the nervous and immunological systems
- The enteric nervous system and the immune system of the gastrointestinal tract communicate in two ways: transmitters released by enteric neuron terminals in the mucosa influence immune-related cells, such as mast cells, and mucosal cells release active substances, such as cytokines and mast cell tryptase, that act on enteric neurons (De Giorgio et al. 2004; Lomax et al. 2006). Inter-communication in illnesses such as Crohn’s disease and ulcerative colitis is complex and beyond the scope of this brief discussion.
FAQ
What is the location of the enteric nervous system (ENS)?
The enteric nervous system (ENS) is a network of sensory neurons, motor neurons, and interneurons that runs from the lower third of the esophagus to the rectum in the gastrointestinal tract.
What is the role of the enteric nervous system and where is it located?
The enteric nervous system (ENS) is found in the digestive tract. It is a sensory, motor, and interneuronal network that runs from the esophagus to the rectum.
What are the two portions of the enteric nervous system (ENS)?
In the peripheral nervous system, the enteric nervous system (ENS) is both the largest and most compound division. The myenteric (Auerbach) and submucosal (Meissner) plexuses are the two basic components of the ENS.
What are the enteric nervous system’s primary roles?
The enteric nervous system (ENS) is a semi-autonomous component of the nervous system that controls muscular activities, local blood flow, mucosal transport, and secretions, as well as immunological and endocrine functions.
What else do you call the enteric nervous system?
The enteric neural system (ENS), also known as the intrinsic nervous system, is a branch of the autonomic nervous system that regulates digestion. It is a network of about 100 million nerve cells (neurons) that run from the esophagus to the anus.
What is an illustration of the enteric nervous system?
EXAMPLES. When we are frightened or stressed, the second brain of the enteric nervous system causes us to get butterflies in our stomach or need to use the loo more frequently. The enteric nervous system (ENS) is distinct from the digestive (GI) system.
What is the significance of the term “second brain” for the enteric nervous system?
The enteric nervous system, which regulates our intestines, is frequently referred to as the body’s “second brain.” Although it cannot write poetry or answer mathematics, this vast network employs the same chemicals and cells as the brain to aid digestion and warn the brain when anything is wrong.
What kind of neurons are there in the enteric system?
Local and centrally projecting sensory neurons monitor mechanical and chemical conditions in the gut, local circuit neurons integrate this information, and motor neurons influence the activity of the smooth muscles in the gut wall and glandular secretions.
Is the enteric nervous system classified as CNS or PNS?
The enteric neural system (ENS) is a component of the peripheral nervous system (PNS) that regulates gastrointestinal behavior without the need for input from the central nervous system (CNS).
What are the advantages of the enteric nervous system?
Because of local reflex circuits, the ENS can function both with and without input from the central nervous system. The ENS performs a variety of activities, including food propulsion, nutrient processing, blood flow modulation, and immunological defense.
What are the three distinct parts of the nervous system?
The central nervous system, the peripheral nervous system, and the autonomic nervous system comprise the human nervous system. It is, without question, the most complicated part of the human body, a natural marvel.
Are the ENS domains secure?
ENS domains are regarded as more secure and private than the Internet’s DNS because they are built on smart contracts.